Mastering Molarity and Dilutions Worksheet Answers

If you've ever grappled with the concepts of molarity and dilutions in chemistry, you're not alone. Mastering these fundamental topics is essential for understanding solutions and their behaviors. This blog post aims to delve into molarity and dilutions by providing comprehensive answers to common worksheet questions, thereby enhancing your understanding of these crucial aspects of chemistry.
Understanding Molarity

Molarity (M) is a measure of the concentration of a solute in a solution. It is defined as the number of moles of solute per liter of solution. Here’s the formula to calculate molarity:
Molarity (M) = moles of solute / volume of solution in liters
Why is Molarity Important?

- Quantitative Measurement: Molarity provides a way to quantify how much solute is dissolved in a solvent, which is crucial for experiments where precise concentrations are necessary.
- Reactions and Stoichiometry: Knowing the molarity helps in calculating the amount of substances involved in chemical reactions, enabling accurate predictions of reaction outcomes.
- Pharmaceutical Industry: Molarity is used to formulate and control the strength of pharmaceutical solutions.
Examples of Molarity Calculations

Let’s walk through some common molarity problems to understand its application:
Example 1: Direct Calculation

If you have 0.5 moles of sodium chloride (NaCl) dissolved in 2 liters of water, what is the molarity?
- Using the formula: M = moles / volume
- M = 0.5 mol / 2 L = 0.25 M
Example 2: Reverse Calculation

Calculate the moles of solute needed to prepare a 0.2 M solution with 1 liter of water.
- From the formula, rearrange for moles: moles = M × volume
- moles = 0.2 M × 1 L = 0.2 mol
🔍 Note: When preparing solutions, ensure you measure volumes at the temperature at which the solution will be used because volume changes with temperature, affecting molarity.
Dilution of Solutions

Dilution is the process of reducing the concentration of a solute in a solution, typically by adding more solvent. Here’s how you manage dilutions:
C₁V₁ = C₂V₂
How to Perform a Dilution

- Calculate: Use the formula above where C₁ and V₁ represent the initial concentration and volume, and C₂ and V₂ are the final concentration and volume.
- Add Solvent: Add the required amount of solvent to dilute the solution.
- Mix: Ensure the solution is thoroughly mixed to maintain homogeneity.
Example of Dilution

If you have a 1 M solution of hydrochloric acid (HCl) and you want to dilute it to 0.1 M in 500 mL of final solution:
- C₁ = 1 M, V₁ = X, C₂ = 0.1 M, V₂ = 500 mL (0.5 L)
- 1 * X = 0.1 * 0.5 L
- X = 0.1 * 0.5 / 1 = 0.05 L or 50 mL
- You would need 50 mL of the 1 M HCl and then add 450 mL of water to make 500 mL of 0.1 M HCl.
Mastering Dilutions Worksheet Answers

Here are some worksheet answers to common dilution problems:
Problem 1
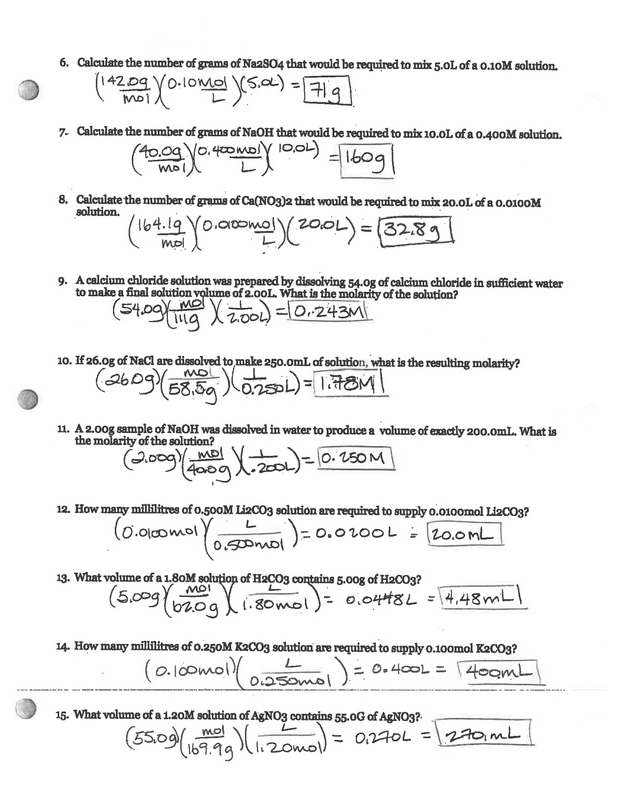
How much water must be added to 20 mL of 10% v/v ethanol solution to produce a 5% v/v ethanol solution?
- Original: C₁V₁ = (10⁄100)V₁
- Diluted: C₂V₂ = (5⁄100)(V₁ + Vwater)
- (10⁄100)*20 = (5⁄100)(20 + Vwater)
- 2 = (20 + Vwater)/20
- 40 = 20 + Vwater
- Vwater = 20 mL
Problem 2

How would you prepare 250 mL of a 0.5 M solution from a stock of 2 M solution?
- C₁V₁ = C₂V₂
- 2 M * V₁ = 0.5 M * 250 mL
- V₁ = 62.5 mL (This is the volume of stock solution needed)
- Therefore, you need 62.5 mL of 2 M stock and 187.5 mL of solvent to make the desired solution.
💡 Note: Always mix solutions in a clean container to avoid contamination, which could affect the accuracy of your dilution.
Summary

In this blog post, we’ve explored the intricacies of molarity and dilutions, with step-by-step solutions to typical worksheet problems. Mastering these concepts involves:
- Understanding the definition and calculation of molarity.
- Applying the dilution formula correctly.
- Practicing various dilution and molarity problems to gain confidence.
By following these guidelines and practicing with real-world examples, you can enhance your proficiency in handling solutions in chemistry, which is vital for both academic and practical applications.
What is the difference between molarity and normality?

+
Molarity refers to the number of moles of solute per liter of solution, while normality is the number of gram equivalents of solute per liter of solution. Normality depends on the chemical reaction involved and can change based on the reaction’s stoichiometry.
Why might one choose to use dilutions?

+
Dilutions are often used to make stock solutions manageable for experimental purposes, to reduce the concentration of a substance for safety or practical reasons, or to prepare solutions at specific concentrations for analytical methods like spectroscopy or titration.
How accurate do dilutions need to be in lab settings?

+
The accuracy of dilutions in lab settings depends on the application. For qualitative analysis, slight deviations might be acceptable, whereas quantitative analysis requires high precision, often necessitating the use of precise measurement tools like pipettes.