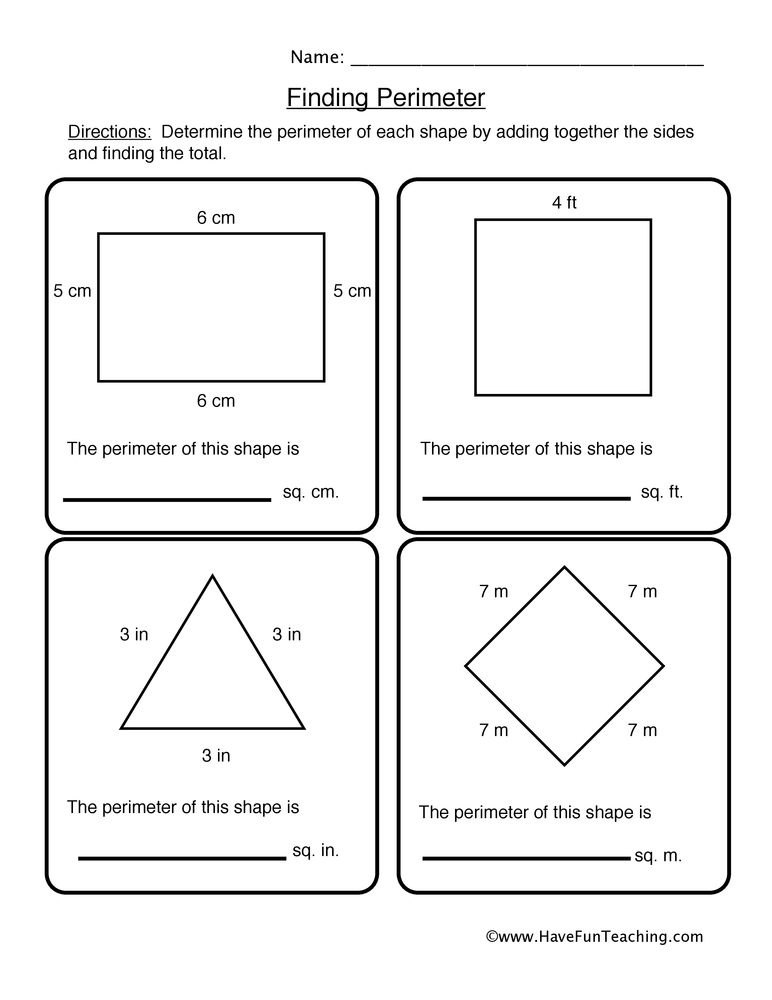
Unlock Answers: Matter and Thermal Energy Worksheet Explained

Discovering how matter and thermal energy interact can be fascinating and often complex, especially when you're delving into a worksheet full of questions that demand understanding and sometimes a bit of deeper research. In this blog post, we will provide comprehensive answers and explanations to common questions about matter and thermal energy, tailored to enhance your learning and comprehension. Let's dive into these fundamental physics concepts with curiosity and clarify every inch of the topic.
What is Matter?
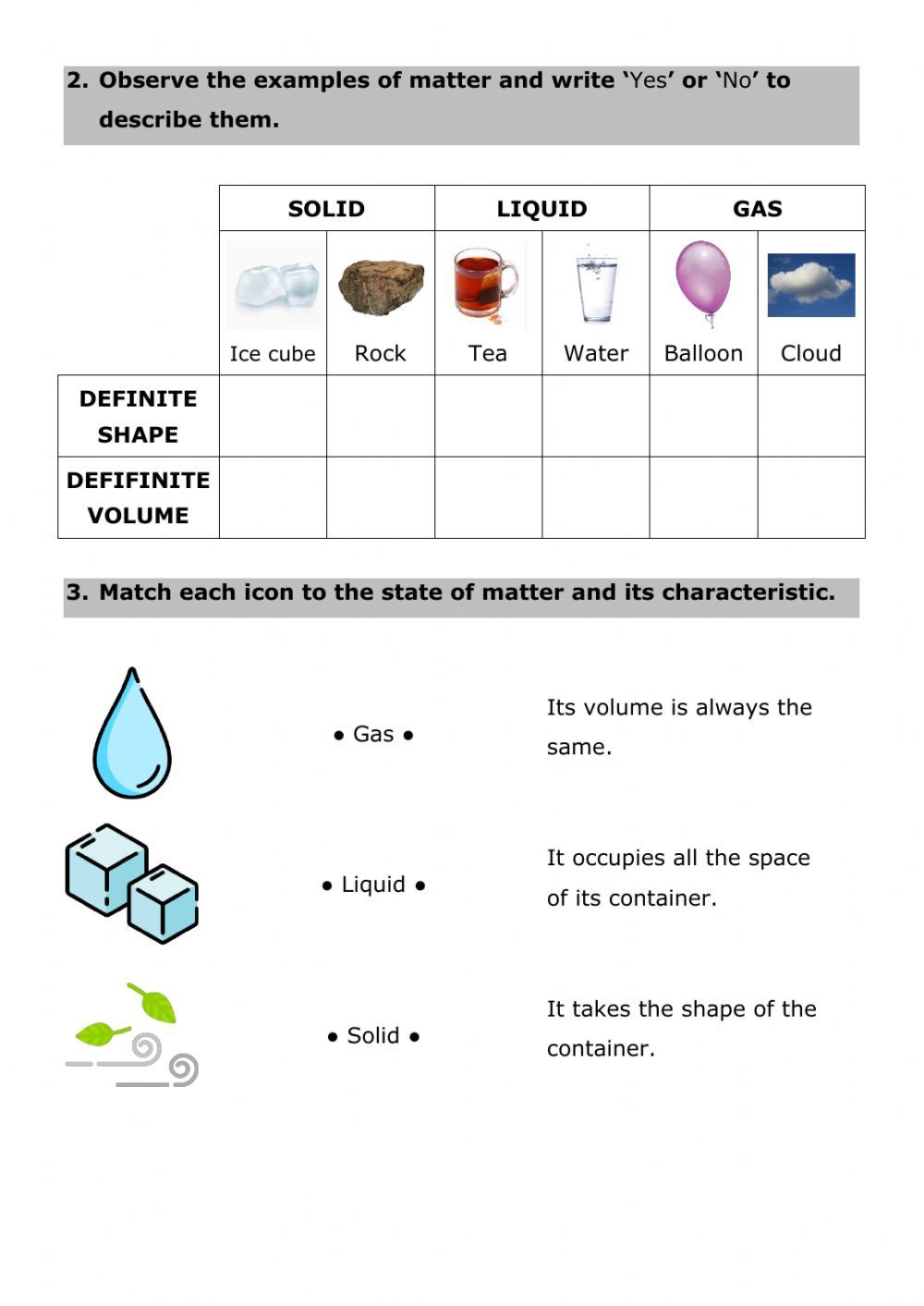
Matter is anything that occupies space and has mass. It can be found in several states, commonly known as:
- Solid: Fixed shape and volume. E.g., ice.
- Liquid: Fixed volume but not shape. E.g., water.
- Gas: Neither fixed shape nor volume. E.g., steam.

Defining Thermal Energy

Thermal energy, often referred to as heat, is a form of energy arising from the motion of particles (atoms or molecules) within an object or system. This energy increases as temperature rises because:
- The particles move faster.
- Their average kinetic energy increases.

How Matter and Thermal Energy Interact

The interaction between matter and thermal energy is multifaceted. Here are some key interactions:
- Expansion and Contraction: When matter is heated, it generally expands due to the increased energy of the particles. Conversely, cooling leads to contraction.
- Phase Changes: Heat can cause matter to undergo phase transitions between solid, liquid, and gas. For example, ice melts into water when it absorbs enough heat.
- Thermal Expansion: Different materials expand at different rates, which can lead to interesting phenomena like bimetallic strips.
| Material | Linear Expansion Coefficient |
|---|---|
| Aluminum | 0.000023 per °C |
| Iron | 0.000012 per °C |

🔬 Note: These coefficients are crucial when designing structures or objects subject to temperature changes.
Worksheet Problem-Solving

Let’s address some typical worksheet problems related to matter and thermal energy:
- Problem: Explain why a glass container might break when filled with hot water?
- The inside of the glass heats up much faster than the outside.
- This temperature differential creates a mechanical stress due to different rates of thermal expansion. If the stress exceeds the glass’s tensile strength, it will crack or shatter.
- Problem: Calculate the amount of energy needed to melt 1 kg of ice at 0°C?
The glass, particularly if it’s thick and borosilicate glass, might not withstand the rapid temperature change. Here’s why:
Using the heat of fusion for ice, which is 334 kJ/kg, the calculation would be:
Energy = Mass × Heat of fusion = 1 kg × 334 kJ/kg = 334 kJ
🧪 Note: This energy just initiates the phase change; further energy is needed to raise the temperature of the water from 0°C to its desired temperature.
Thermal Energy Transfer

Thermal energy can be transferred through three main mechanisms:
- Conduction: Direct transfer of heat through the collision of particles. More efficient in solids.
- Convection: Transfer due to the movement of a fluid (liquid or gas). Think of boiling water or heat rising from a radiator.
- Radiation: The transfer of heat through electromagnetic waves, like infrared rays from the sun.
Thermal Equilibrium

When two bodies of different temperatures are in contact, heat flows from the hotter to the cooler one until they reach the same temperature, a state known as thermal equilibrium. Here are some points to consider:
- The rate of heat transfer depends on the temperature difference and the material properties.
- Thermal equilibrium does not necessarily mean the bodies have the same amount of thermal energy; only their temperatures are the same.
Understanding these principles can not only help you with your worksheet but also with real-life scenarios involving heating, cooling, and insulation. Whether you're looking to improve the efficiency of a building, the design of a cooking appliance, or merely curious about how our environment functions, thermal energy and matter interactions are fundamental to consider.
How does the mass of an object affect its thermal energy?

+
The mass of an object does not directly increase or decrease its temperature, but it does affect the amount of thermal energy it can store. The more mass, the more thermal energy (or heat) is required to change its temperature by a given amount.
Why do we need to know about the expansion coefficients?

+
Expansion coefficients are crucial in engineering and design because they predict how materials will change size with temperature. This knowledge helps in creating structures or devices that can safely handle temperature variations without cracking or excessive deformation.
Can thermal energy flow without a medium?

+
Yes, through the process of radiation, thermal energy can travel through the vacuum of space. Heat from the sun reaches Earth through radiation because electromagnetic waves do not need a medium to travel through.
The interplay between matter and thermal energy underpins many natural phenomena and technological advancements. With this understanding, your next steps in exploring physics or tackling similar worksheets will be more insightful. Remember, these principles aren’t just theoretical; they are observable and applicable in everyday life. Keep this knowledge in mind, and you’ll see the world through a different, more informed lens.