5 Tips for Mastering Lewis Structure Worksheet 3

Mastering Lewis structures is crucial for understanding chemistry at the molecular level. These diagrams help us visualize the arrangement of atoms and electrons in molecules, aiding in predicting molecular geometry, bond angles, and much more. Here are five detailed tips to excel in completing Lewis Structure Worksheets, particularly when tackling Worksheet 3.
Understand Atomic Structures
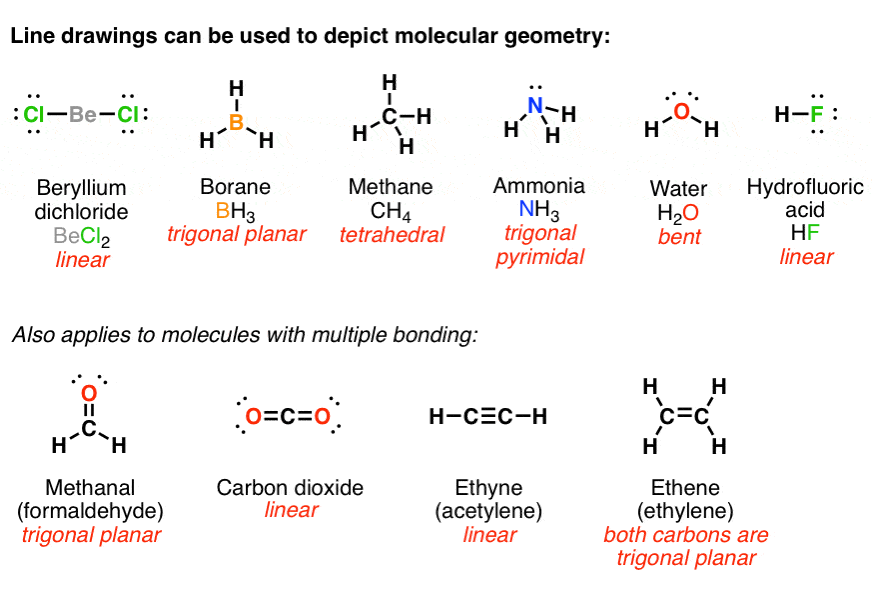
Before diving into drawing Lewis structures, ensure you’re well-versed with the periodic table and atomic structures:
- Valence Electrons: Recognize how many valence electrons each element has. Valence electrons are involved in bonding and can be found in the outermost energy level of an atom.
- Group Trends: Elements in the same group (vertical column) typically have the same number of valence electrons, which simplifies predicting electron configurations.
Learn Electron Count and Placement

Lewis structures rely heavily on electron placement:
- Count Electrons: Add up all the valence electrons from each atom. Don’t forget to consider charge if the molecule is an ion.
- Placement: Place electrons in pairs on the atoms around the central atom, then add extra pairs as lone pairs to complete the octet rule for atoms other than hydrogen.
Focus on the Central Atom
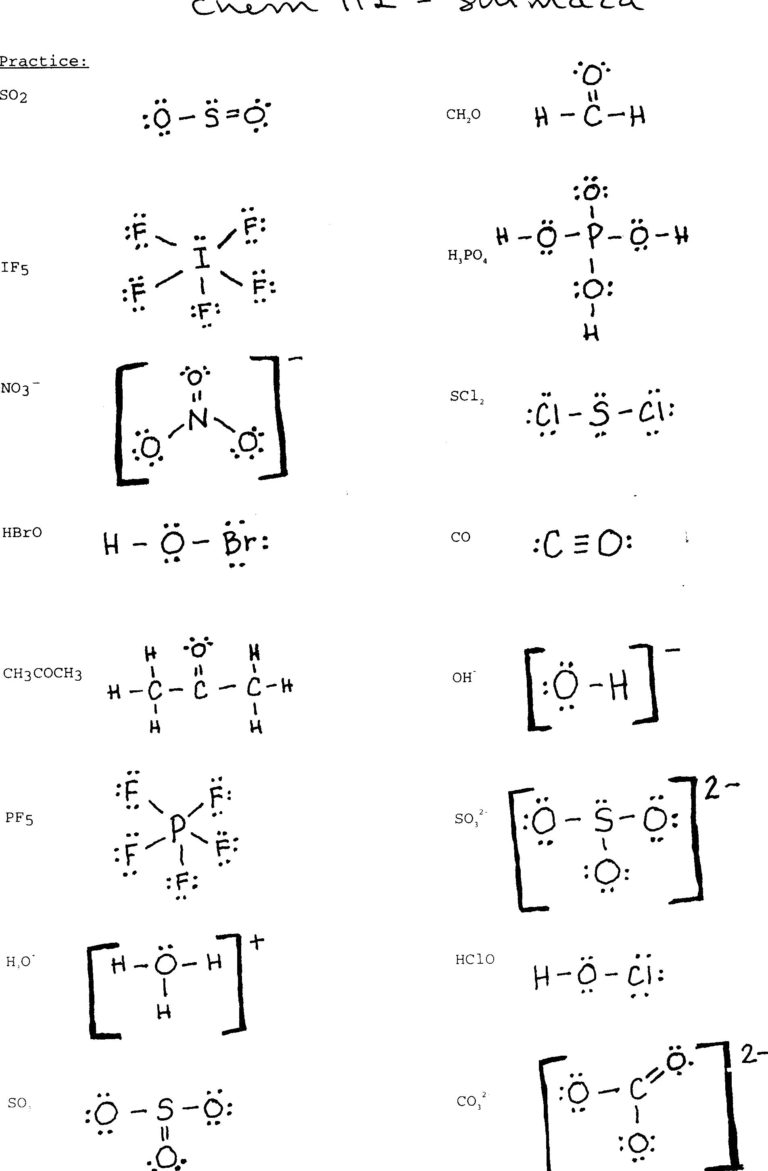
Selecting the correct central atom is key:
- Identify: Choose an atom from the least electronegative group (often C, N, S, or P) to be the central atom. This is where other atoms bond to.
- Connect: Use single bonds to connect all other atoms to the central atom first, then adjust for multiple bonds or lone pairs if necessary.
Use Octet Rule Exceptions

Not all atoms obey the octet rule:
- Hydrogen: Only needs two electrons to complete its outer shell.
- Boron: Often exhibits less than an octet, like in BF3, where Boron has only six electrons.
- Phosphorus and Sulfur: Can expand their octets to accommodate more than eight electrons due to d-orbital availability.
🔬 Note: Understanding when to apply these exceptions is crucial for accurately drawing Lewis structures.
Double and Triple Bonds
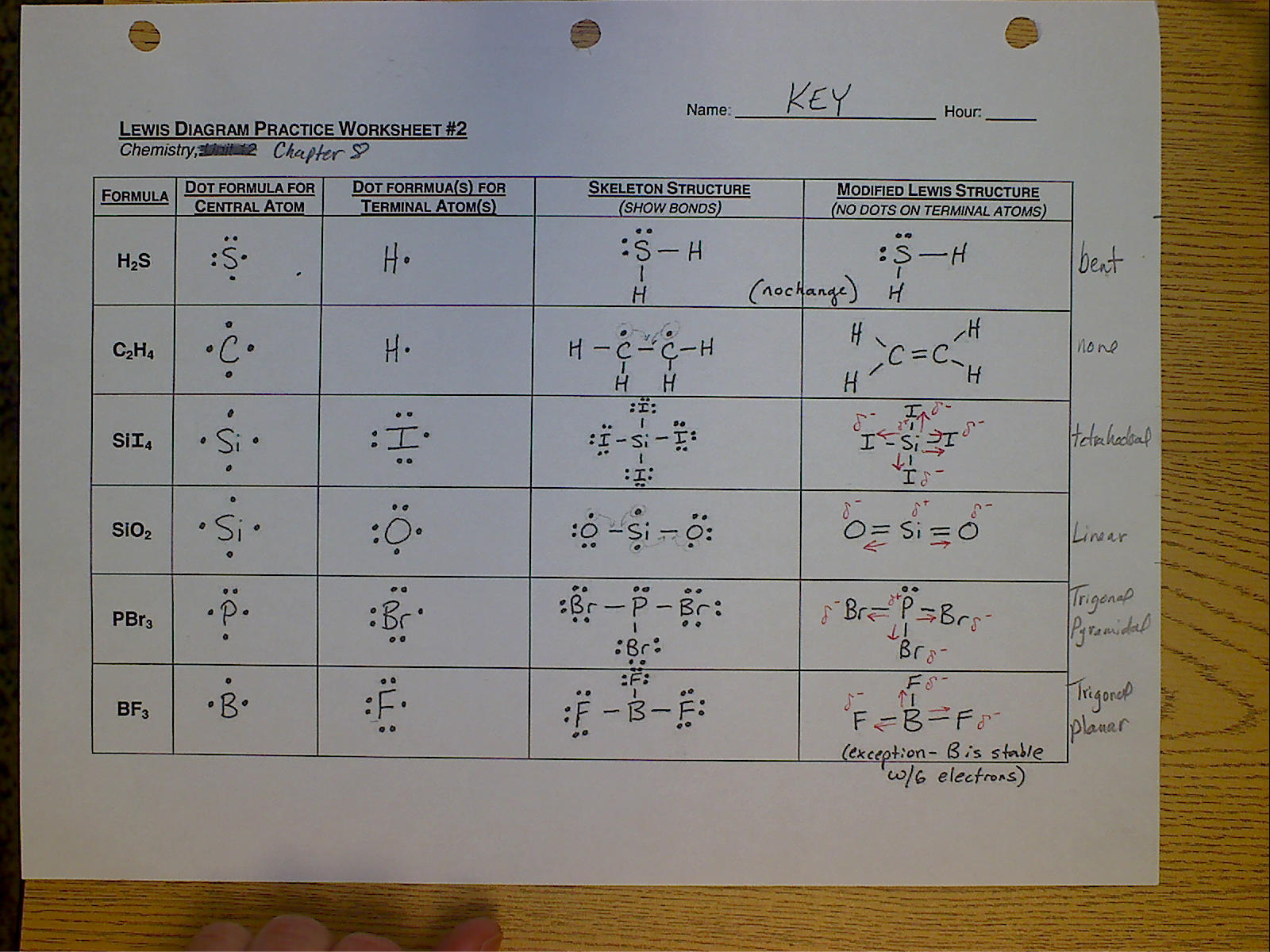
Adjust bonds to meet octet requirements:
- When necessary: If single bonds aren’t sufficient to fulfill octets, form double or triple bonds by moving lone pair electrons.
- Check Stability: Ensure that the structure is stable and that all atoms have the appropriate electron count.
Summing up, mastering Lewis structures isn't just about memorizing how to draw them. It’s about understanding the fundamental principles of chemistry, the nature of bonds, electron distribution, and atomic behavior. With these five tips in mind, you're better equipped to tackle Worksheet 3 and beyond, enhancing your ability to analyze chemical structures with precision and confidence.
Why are Lewis structures important?

+
Lewis structures help chemists visualize how electrons are shared between atoms in a molecule, enabling predictions about chemical behavior, including reactivity and bonding patterns.
How do you know when to use double or triple bonds?

+
When single bonds don’t provide enough electrons to complete octets or achieve formal charge neutrality, you’ll form double or triple bonds by sharing additional pairs of electrons between atoms.
What if an atom has more than eight electrons?

+
Atoms from the third period and beyond can have more than eight electrons by using d-orbitals, allowing for expanded octets in compounds like PCl5 or SF6.



