Master Lewis Dot Structures: Easy Worksheet Guide
Understanding Lewis dot structures can be a transformative part of your chemistry education journey. These diagrams provide a visual representation of the bonding between atoms, allowing you to better comprehend how atoms share electrons to form stable molecules. If you've ever found yourself puzzled by molecular structure or bonding, this guide will help you master Lewis dot structures through an easy-to-follow worksheet approach. Let's delve into this fundamental concept step-by-step, ensuring that you're equipped with the knowledge to tackle any chemistry challenge.
What Are Lewis Dot Structures?


Lewis dot structures, also known as Lewis structures or electron dot structures, were developed by Gilbert N. Lewis. They are a simplified way of showing how atoms in a molecule or ion are bonded. Here's a quick breakdown:
- Each dot represents one electron.
- Bonds are typically shown as lines connecting atoms.
- Only valence electrons (those in the outermost shell) are considered.
Steps to Draw Lewis Dot Structures

Here's how you can systematically draw Lewis dot structures:
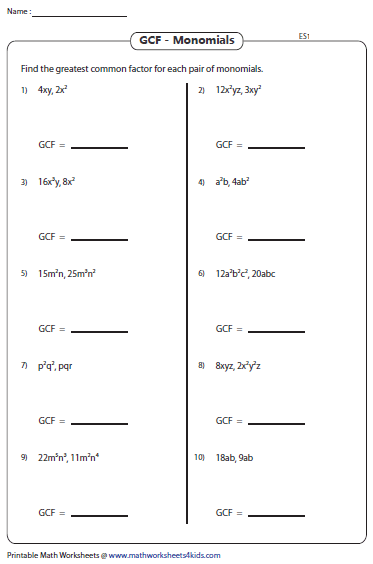
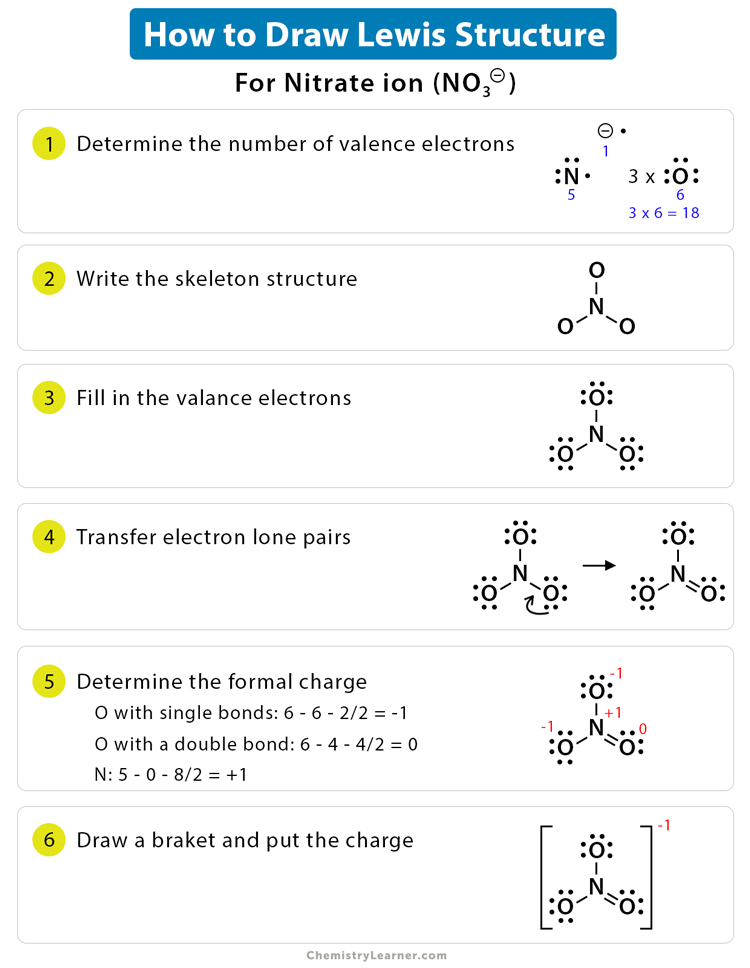
Step 1: Count Valence Electrons

Start by determining the total number of valence electrons in your molecule or ion. Here’s how:
- Sum up the group number of each element from the periodic table. For ions, adjust for the charge by adding (for negative ions) or subtracting (for positive ions) electrons.
Step 2: Choose the Central Atom

Identify the least electronegative atom as the central atom unless:
- Hydrogen is almost always terminal.
- Oxygen is usually terminal except in peroxide structures.
Step 3: Draw Single Bonds

Place the central atom in the center of your structure and connect other atoms to it using single lines to represent bonds. Each bond accounts for two electrons.
Step 4: Distribute Remaining Electrons

After bonding, distribute the remaining electrons to the peripheral atoms first. Remember:
- Most atoms achieve an octet (8 electrons) except hydrogen (2) and helium (2).
- Elements like boron might achieve only six electrons, forming structures like BF3.
Step 5: Check for Formal Charges

Ensure that the charges on individual atoms make sense. Here’s how:
- Formal Charge = Valence Electrons - (Nonbonding Electrons + ½ Bonding Electrons)
- Try to minimize formal charges by forming double or triple bonds where possible.
Step 6: Finalize the Structure

If necessary, adjust your structure to minimize formal charges:
- Forming double or triple bonds by sharing more electrons between adjacent atoms.
- Ensuring that the formal charges are as close to zero as possible.
🌟 Note: Practice drawing Lewis structures for simple molecules like H2O, CO2, and NH3 to get comfortable with these steps.
Common Mistakes to Avoid

- Overcounting or Undercounting Electrons: Double-check your count against the total valence electrons.
- Misplacing Lone Pairs: Distribute electrons to achieve the octet rule where applicable.
- Incorrect Central Atom: Often, the least electronegative atom should be central, not counting hydrogen or oxygen.
Worksheet Practice

To solidify your understanding, let’s go through a practical example:
| Molecule | Lewis Structure |
|---|---|
| H2O |
O:
H:O:H
|
| NH3 |
H H
| | |
N:
|
H
|
| CO2 |
O=C=O
|

As you practice, remember these tips:
- Always start with the count of valence electrons.
- Consider the octet rule and exceptions like BF3 or certain transition metals.
- Adjust for formal charges if necessary.
Mastering Lewis dot structures isn't just about following rules; it's about understanding the underlying chemistry. These diagrams help predict molecular shapes, polarity, and reactivity, making them indispensable in any chemist's toolkit.
As we've covered, mastering Lewis dot structures involves following a set of systematic steps, understanding common pitfalls, and lots of practice. By internalizing these steps and practicing with real molecules, you'll be well on your way to understanding not only chemical bonding but also how these structures inform the properties of molecules and their reactions. This guide has equipped you with the foundational knowledge to approach any molecule confidently, making sense of how atoms bond and interact at a fundamental level.
Why do we need to know Lewis dot structures?
+
Understanding Lewis dot structures helps you predict molecular properties like shape, polarity, and reactivity, which are essential for various chemical applications.
<div class="faq-item">
<div class="faq-question">
<h3>Can all compounds be represented by Lewis dot structures?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>Not all compounds can be perfectly described with Lewis structures, especially those involving transition metals, elements with d or f orbitals, or extremely complex molecules. However, it's a good starting point for most simple covalent compounds.</p>
</div>
</div>
<div class="faq-item">
<div class="faq-question">
<h3>What's the significance of formal charges in Lewis structures?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>Formal charges help identify the most stable resonance structure of a molecule or ion. They indicate how electron distribution might deviate from a molecule's neutral state, influencing molecular stability.</p>
</div>
</div>