Mastering Formal Charge Calculations: Essential Worksheet Included

Introduction to Formal Charge

In the realm of chemistry, understanding molecular structures and their stability often hinges on mastering the calculation of formal charges. This crucial tool helps chemists predict the most likely arrangement of atoms within a molecule, thus guiding experiments and theoretical predictions. Formal charge calculation is not just about numbers; it’s an insight into the electron distribution, providing a window into reactivity, polarity, and much more. Let’s delve into this topic to ensure you’re well-equipped to tackle these calculations with confidence.
Why Calculate Formal Charges?

Formal charges are not merely an academic exercise; they serve several practical purposes:
- Stability Analysis: Helps identify the most stable resonance structure in a molecule.
- Bonding Prediction: Aids in predicting where bonds might form or break within a molecule.
- Reactivity: Indicates potential sites for nucleophilic or electrophilic reactions.
- Molecular Polarity: Offers clues about the overall dipole moment of a molecule.
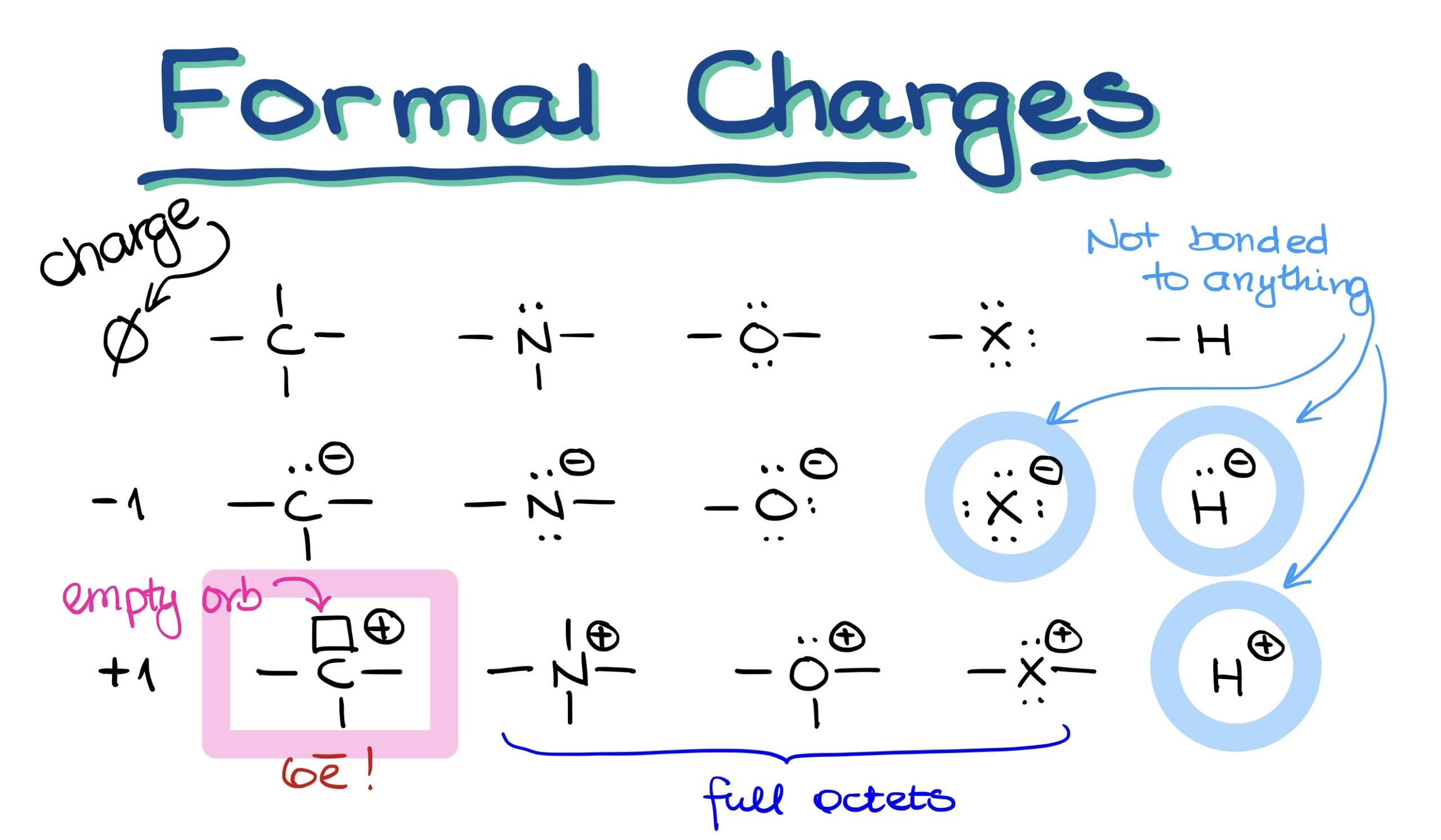
How to Calculate Formal Charges

The formal charge on an atom can be calculated using the following formula:
Formal Charge = V - (L + 1⁄2 * B)
- V = Total number of valence electrons in the free atom
- L = Total number of non-bonding electrons (lone pairs) on the atom
- B = Total number of bonding electrons divided by 2
Practical Examples
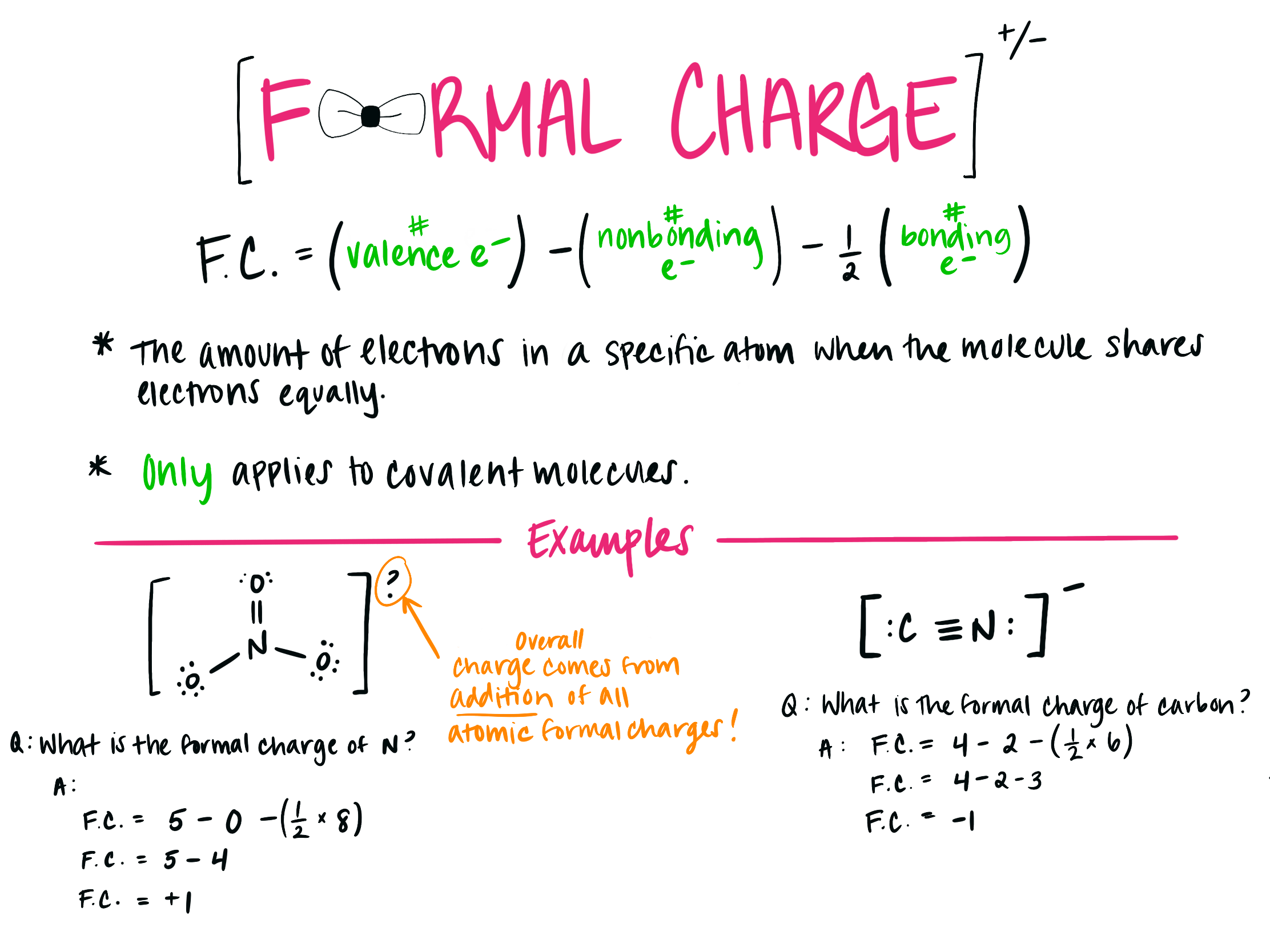
Let’s work through a few examples to illustrate how to apply this formula:
Example 1: Carbon Dioxide (CO2)

Here’s how to calculate formal charges for CO2:
| Atom | V | L | B | Formal Charge |
|---|---|---|---|---|
| C | 4 | 0 | 8 | 4 - (0 + 4) = 0 |
| O (each) | 6 | 4 | 2 | 6 - (4 + 1) = +1 |
⚗️ Note: In CO2, the formal charges indicate the stability of the structure with carbon having a zero formal charge and oxygen atoms each having a +1 formal charge, which aligns with experimental observations.
Example 2: Ammonia (NH3)

For NH3:
| Atom | V | L | B | Formal Charge |
|---|---|---|---|---|
| N | 5 | 2 | 6 | 5 - (2 + 3) = 0 |
| H (each) | 1 | 0 | 2 | 1 - (0 + 1) = 0 |
Formal Charge Worksheet

Here’s a worksheet to help you practice:
| Molecule | Atom | V | L | B | Formal Charge |
|---|---|---|---|---|---|
| Water (H2O) | O | 6 | 4 | 4 | 6 - (4 + 2) = 0 |
| Water (H2O) | H | 1 | 0 | 2 | 1 - (0 + 1) = 0 |
Final Thoughts

As we wrap up our exploration into formal charges, remember that this skill is not just about calculating numbers but understanding the underlying principles of how molecules interact and behave. Formal charges are like pieces of a puzzle that help us predict and explain the structure, stability, and reactivity of compounds. They guide chemists in synthesizing new molecules, analyzing existing ones, and understanding molecular behavior at a microscopic level.
Why is it important to calculate formal charges?

+
Formal charges help predict the most stable arrangement of atoms in a molecule, thus offering insights into chemical reactions, bonding, and molecular behavior.
Can an atom have a formal charge of zero?
+
Yes, if the number of valence electrons in the free atom equals the sum of lone pair electrons and half the shared electrons.
How does the formal charge affect the stability of a molecule?

+
A structure with formal charges closer to zero and negative charges on more electronegative atoms tends to be more stable.