Ionic vs. Covalent Bonds: 5 Key Differences Visualized

Chemical bonding, the union of two or more atoms to form compounds, is a fundamental concept in chemistry. Among the various types of bonds, ionic and covalent bonds are the most prevalent. Here, we will dive into the 5 key differences between these bond types, visualizing their unique characteristics and implications.
1. Formation Mechanism

Ionic Bonds:
- Ionic bonds occur between a metal and a non-metal.
- Atoms engage in electron transfer where one atom loses an electron (becoming a cation) and another gains it (becoming an anion).
- The resulting attraction between these oppositely charged ions leads to the formation of an ionic bond.
Covalent Bonds:
- Covalent bonds form primarily between non-metals.
- Atoms share electrons to achieve stability and complete their outermost electron shell.
- The shared pair of electrons orbits around both bonded nuclei.
| Type | Formation |
|---|---|
| Ionic | Electron transfer leading to charge attraction |
| Covalent | Electron sharing to complete electron shells |

🔧 Note: Ionic bonds involve electron transfer, while covalent bonds involve electron sharing.
2. Structure and Properties

Ionic Compounds:
- Form crystalline structures with a regular repeating pattern.
- High melting and boiling points due to strong electrostatic attractions.
- Conduct electricity when molten or dissolved in water but not in solid form.
Covalent Molecules:
- Exist as individual molecules or as macromolecules (like diamond or graphite).
- Generally have lower melting and boiling points; exceptions exist in network covalent compounds.
- Non-conductive in all states due to no free-moving charge carriers.
3. Nature of Bonding

Ionic Bonds:
- The bond is electrostatic in nature, resulting from attraction between ions with opposite charges.
- The bond strength is high, leading to rigid, brittle substances.
Covalent Bonds:
- Result from the mutual attraction of atomic nuclei for a shared pair of electrons.
- The strength of the bond varies, but covalent compounds can be flexible or malleable.
4. Electronegativity and Polarity
Ionic Bonds:
- A significant difference in electronegativity between atoms leads to ionic bonding.
- The polarity of these bonds is maximal due to the complete electron transfer.
Covalent Bonds:
- Can be polar or non-polar depending on the electronegativity difference between bonded atoms.
- A small electronegativity difference results in a non-polar covalent bond, while a larger difference leads to a polar covalent bond.
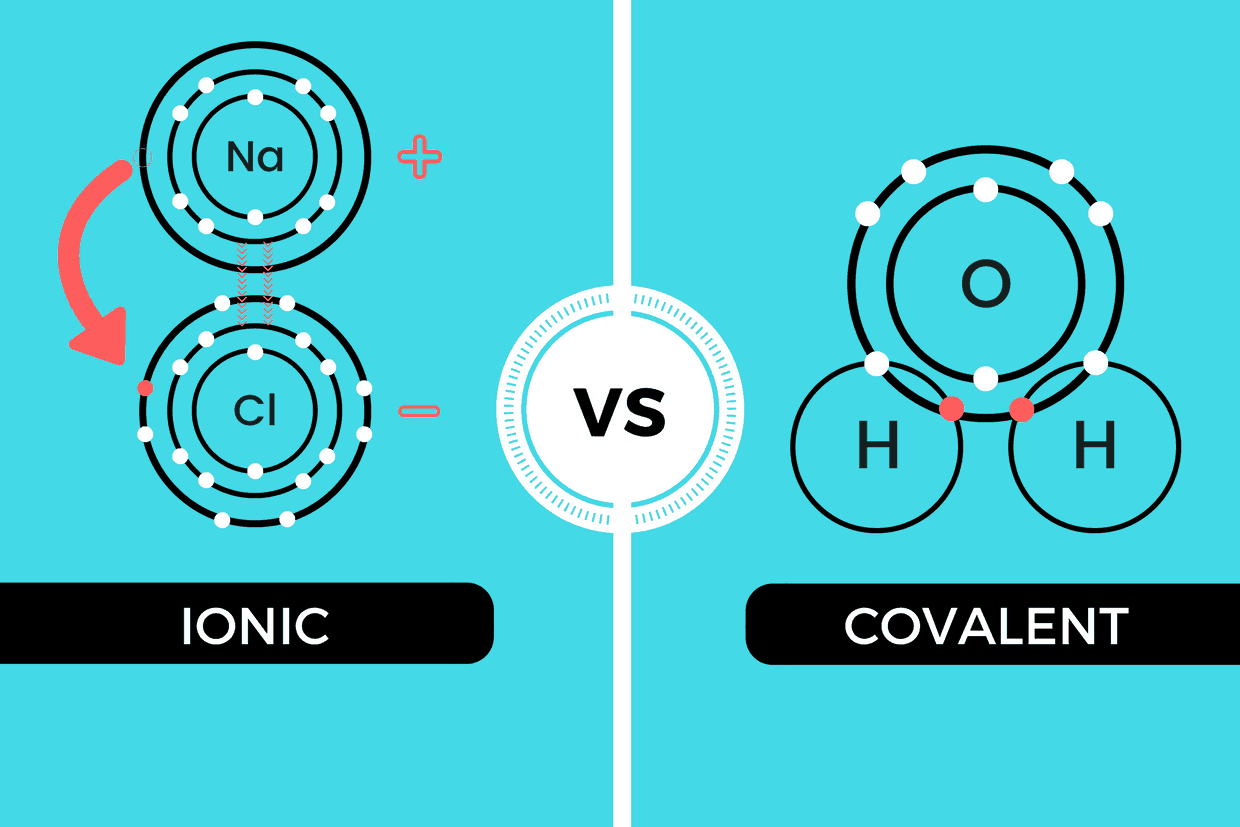
5. Examples and Visualization

Here's how we can visualize these bonds:
- Ionic: Sodium chloride (NaCl) - sodium donates an electron to chlorine to form Na+ and Cl-, which are then attracted to each other.
- Covalent: Water molecule (H2O) - oxygen shares electrons with two hydrogen atoms to complete their shells.
🔎 Note: The nature of chemical bonds can range from purely ionic to purely covalent with various degrees of ionic character in covalent bonds.
Understanding these differences helps chemists predict the behavior of compounds in various chemical reactions. Ionic compounds tend to be salts, dissolved in water or forming high-melting point solids. Covalent compounds can range from gases, like oxygen, to complex macromolecules like proteins. The polarity of covalent bonds also affects solubility, reactivity, and molecular geometry, making it a crucial factor in understanding chemistry.
Can ionic and covalent bonds exist within the same molecule?

+
Yes, compounds can exhibit both types of bonding; for instance, in complex molecules like amino acids where peptide bonds involve both covalent bonds between atoms and ionic interactions between groups.
What is the significance of electronegativity in chemical bonding?

+
Electronegativity determines the nature of the bond (ionic, polar covalent, or non-polar covalent) and influences chemical properties like bond strength, polarity, and reactivity.
Why do ionic compounds conduct electricity in solution?

+
Ionic compounds dissolve in water into free ions, which are charged particles that carry electricity through the solution.
Are ionic compounds soluble in non-polar solvents?

+
Typically, ionic compounds are not soluble in non-polar solvents due to the lack of interaction between the ions and the non-polar solvent molecules.
How does covalent bonding affect the boiling and melting points?

+
Covalent compounds often have lower melting and boiling points because the intermolecular forces holding the molecules together are generally weaker than the electrostatic forces in ionic compounds.
The exploration of ionic and covalent bonds reveals the intricate dance of electrons that shapes the world of chemistry. These fundamental differences illustrate how chemistry governs material properties, reactivity, and biological functions, making it an endlessly fascinating field of study.



