Ionic vs. Covalent Bonds: Color Your Way to Chemistry Mastery!

Welcome to the fascinating world of chemical bonding! Chemistry can be both a daunting and thrilling subject, especially when exploring the intricate relationships between atoms. Today, we're diving into the core concepts of ionic and covalent bonds through an engaging and colorful approach—using colored pencils to make sense of these fundamental connections in molecules. Buckle up as we explore how these invisible forces dictate the matter around us.
Understanding Chemical Bonds

Before we color our chemistry sheets, it’s essential to grasp what a chemical bond really is:
- Ionic Bonds: This bond type involves the complete transfer of one or more valence electrons from one atom to another, resulting in the formation of ions. The atom losing electrons becomes positively charged (cation), and the one gaining electrons becomes negatively charged (anion).
- Covalent Bonds: Unlike ionic bonds, covalent bonds involve sharing electron pairs between atoms. These bonds can form between similar or different atoms, creating either simple molecules or large networks.
💡 Note: Both types of bonds aim to achieve electron configuration stability, often striving for the electron count of a noble gas.
Color Your Chemistry: Ionic Bonding


Let’s visualize ionic bonding with colors:
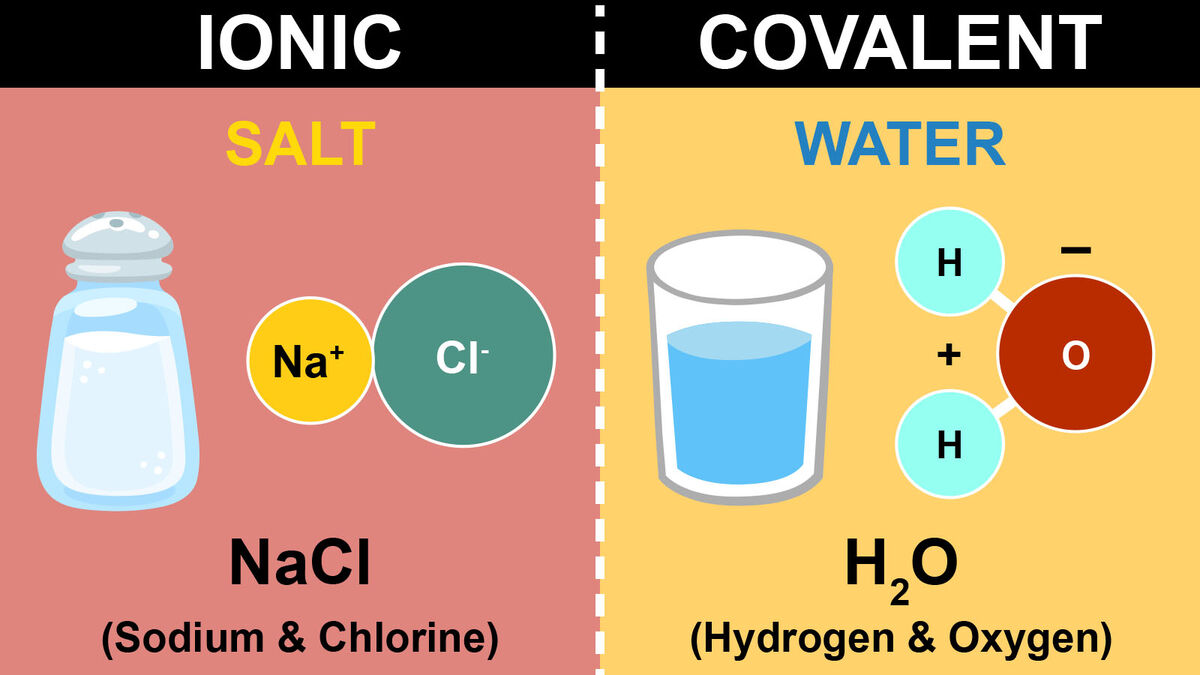
- Start with Sodium Chloride (NaCl): Color sodium (Na) with a bright orange, indicating its positive charge after losing an electron.
- Sodium’s electron configuration: [Na] = [He] 2s2 2p6 (lose 1 electron to have [Ne])
- Sodium Atom:
Atomic Number Protons Electrons Charge 11 11 10 +1 
- Chlorine (Cl): Use a vibrant green for chlorine, which gains that electron to achieve stability.
- Chlorine’s electron configuration: [Cl] = [Ne] 3s2 3p5 (gain 1 electron to have [Ar])
Atomic Number Protons Electrons Charge 17 17 18 -1 - The Bonding Process: Draw arrows in your coloring sheet from Na to Cl, showing the electron transfer.
Covalent Bonding: Sharing is Caring


Now, let’s paint the canvas of covalent bonds:
- Hydrogen Gas (H2): Two hydrogen atoms share their electrons to achieve stability. Color these atoms in a soft blue, representing the shared electron cloud.
- Methane (CH4): One carbon atom shares electrons with four hydrogen atoms. Use carbon in a darker blue and hydrogen in a lighter blue to show how these bonds work.
🖌️ Note: Covalent bonds often result in molecules, where electrons form localized bonds.
Polar Covalent Bonds: A Touch of Polarity

In our journey, we find a nuanced bond type:
- Polar Covalent Bonds: When two atoms have a significant difference in electronegativity, the shared electrons are not equally distributed. For instance:
- H2O (Water): Color oxygen in red to denote its high electronegativity and hydrogen in light blue, with arrows showing the electron pull toward oxygen.
Wrapping Up Your Colorful Chemistry Session

By now, your chemistry sheets should be a vibrant display of how atoms interact through ionic and covalent bonds. Here are the key takeaways:
- Ionic bonds involve electron transfer leading to charged ions.
- Covalent bonds are about sharing electrons, often forming molecules.
- Polar covalent bonds introduce the concept of electronegativity affecting bond polarity.
Understanding these foundational concepts through a creative lens not only aids in memorization but also makes chemistry a more approachable and visual subject. As you continue exploring chemistry, remember that these bonds are the very foundation of the physical world around us.
What is the main difference between ionic and covalent bonds?
+
The primary difference lies in the method of electron interaction. Ionic bonds involve a complete transfer of electrons from one atom to another, creating oppositely charged ions that attract each other. Covalent bonds, on the other hand, involve sharing electrons between atoms, with the aim of achieving a stable electron configuration.
Can a molecule have both ionic and covalent bonds?

+
Yes, some compounds can exhibit both types of bonding. For example, in compounds like ammonium chloride (NH4Cl), you’ll find covalent bonds within the ammonium ion (NH4+), while the entire ion interacts ionically with the chloride ion (Cl-).
How can color help in understanding chemical bonds?

+
Using color allows for visual distinction between different elements and their electron configurations. By associating specific colors with atom types or charges, learners can visually track electron transfers, sharing, or polar interactions, making the abstract concepts more concrete and easier to understand.



