Ionic Compounds Naming and Formulas Worksheet Answers

Understanding the nomenclature of ionic compounds is fundamental for chemistry students. Not only does it help in recognizing and naming substances, but it also aids in predicting the behavior of compounds in various chemical reactions. Here's a comprehensive guide to help you master this topic, complete with exercises and answers.
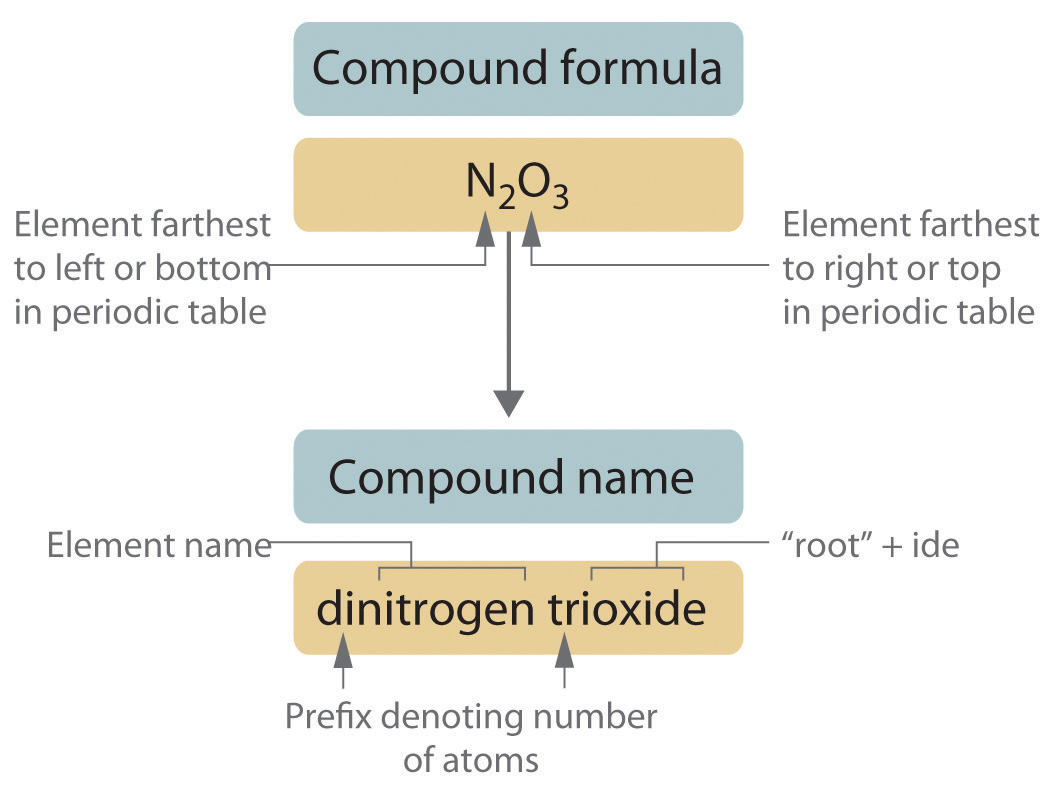
Naming Ionic Compounds: The Basics

Ionic compounds are formed when a metal transfers one or more electrons to a non-metal. The metal becomes positively charged (cation), and the non-metal becomes negatively charged (anion). Here are the steps to name these compounds:
- Identify the cation: Name it the same as the element (e.g., sodium for Na+).
- Identify the anion: Use the stem of the element name and add ‘-ide’ (e.g., chloride for Cl-).
- Combine the names: Write the cation name first, followed by the anion name, without spaces or prefixes (e.g., sodium chloride).

Worksheet and Answers

Here’s a simple worksheet to practice naming ionic compounds:
| Chemical Formula | Compound Name |
|---|---|
| NaCl | Sodium Chloride |
| KI | Potassium Iodide |
| Li2O | Lithium Oxide |
| MgO | Magnesium Oxide |
| CaBr2 | Calcium Bromide |

💡 Note: When dealing with compounds containing transition metals, the charge of the metal can vary. In such cases, use Roman numerals to denote the metal’s charge (e.g., FeCl2 is iron(II) chloride).
Formulas of Ionic Compounds

To derive the formula of an ionic compound, you need to balance the charges so that the compound is electrically neutral. Here are the steps:
- Identify the ions: Know the charges of the ions involved.
- Balance the charges: Find the least common multiple of the charges to balance them.
- Write the formula: Write the cation symbol, followed by the anion symbol with subscripts indicating the number of ions needed for charge balance.
Formulating Ionic Compounds: Worksheet and Answers

Let’s practice deriving chemical formulas from given names:
| Compound Name | Chemical Formula |
|---|---|
| Sodium Chloride | NaCl |
| Potassium Sulfide | K2S |
| Magnesium Oxide | MgO |
| Calcium Phosphide | Ca3P2 |
| Iron(II) Chloride | FeCl2 |
Advanced Concepts in Ionic Compounds

Ionic compounds also include polyatomic ions, where multiple atoms combine to form an ion with a specific charge. Here are some key points:
- Learn common polyatomic ions: They don’t follow the ‘ide’ naming convention (e.g., nitrate (NO3-)).
- Identify the ions: For polyatomic ions, recognize the group of atoms and the overall charge.
- Balance the charges: Similar to simple ions, but polyatomic ions require parentheses when more than one group is needed in the formula (e.g., Ca(NO3)2).
Worksheet and Answers for Polyatomic Ions

Here’s how to write formulas for compounds with polyatomic ions:
| Compound Name | Chemical Formula |
|---|---|
| Sodium Nitrate | NaNO3 |
| Potassium Sulfate | K2SO4 |
| Ammonium Chloride | NH4Cl |
| Magnesium Phosphate | Mg3(PO4)2 |
| Calcium Hydroxide | Ca(OH)2 |
In the spirit of summarizing our journey through the realm of ionic compounds, we’ve explored the essential principles of naming these substances, the rules for writing their formulas, and ventured into the complexities of polyatomic ions. Recognizing and understanding the terminology around ionic compounds not only bolsters our grasp of chemistry but also illustrates the beauty and order in the world of atoms and molecules.
How do you name ionic compounds with transition metals?
+
When naming ionic compounds that contain transition metals, the charge of the metal can vary. Therefore, we use Roman numerals in parentheses to indicate the cation’s oxidation state, e.g., iron(II) chloride (FeCl2). Here, ‘II’ indicates the metal’s charge is +2.
What are polyatomic ions?

+
Polyatomic ions are ions that consist of two or more covalently bonded atoms that together possess a charge. Common polyatomic ions include sulfate (SO42-), nitrate (NO3-), and hydroxide (OH-).
Can ionic compounds exist as molecules?

+
Ionic compounds generally do not form discrete molecules as they exist as extended, three-dimensional networks in their solid state. However, in a solution or when melted, they dissociate into ions, which can form temporary associations that are molecule-like in behavior.



