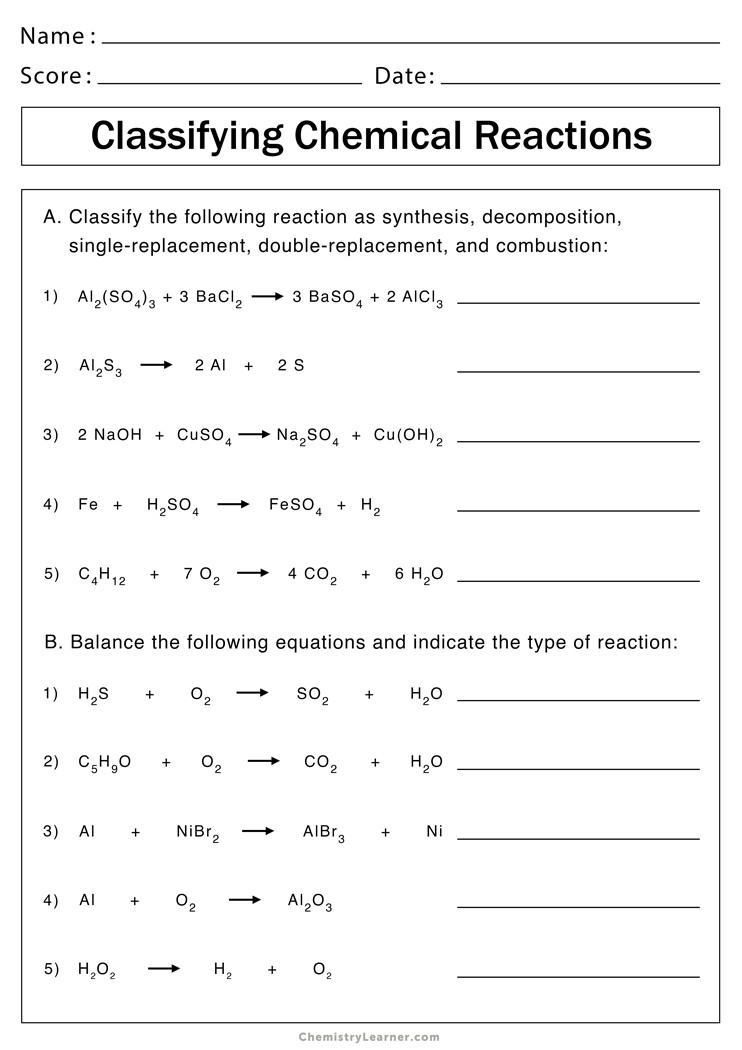
Balancing Chemical Equations: Reaction Types Worksheet Answers

When you're studying chemistry, balancing chemical equations is a fundamental skill you'll encounter frequently. It's not just about making sure that the number of atoms in reactants equals those in the products, but it also helps in understanding reaction mechanisms and stoichiometry. Today, let's dive into the different types of chemical reactions and provide you with some worksheet answers to enhance your comprehension.
Understanding Types of Chemical Reactions

Chemical reactions can be categorized into several types based on their characteristics. Here are the key reaction types:
- Combination or Synthesis Reactions: Where two or more substances combine to form a single compound. The general form is: A + B → AB.
- Decomposition Reactions: These involve breaking down a single compound into two or more elements or new compounds. The general form is: AB → A + B.
- Single Displacement Reactions: One element displaces another in a compound. The equation looks like this: A + BC → AC + B.
- Double Displacement Reactions: Elements from two compounds swap places, forming two new compounds. The form is: AB + CD → AD + CB.
- Combustion Reactions: Typically, oxygen reacts with a fuel, producing carbon dioxide, water, and heat. The simplest equation might look like: CxHy + O2 → CO2 + H2O.
Worksheet: Balancing Chemical Equations by Type

Here’s how you might go about balancing equations for each reaction type:
- Combination Reactions:
Consider the reaction of hydrogen gas with oxygen gas to form water:
2H2 + O2 → 2H2O
🔗 Note: Notice how we multiply hydrogen by 2 to balance oxygen, then water by 2 to balance hydrogen.
- Decomposition Reactions:
When you decompose water back into hydrogen and oxygen:
2H2O → 2H2 + O2
🔗 Note: Here, we see the reactants' coefficients mirroring the products to maintain balance.
- Single Displacement:
Let's say zinc reacts with hydrochloric acid to form zinc chloride and hydrogen:
Zn + 2HCl → ZnCl2 + H2
🔗 Note: The zinc replaces hydrogen, but the hydrogen atoms are balanced by multiplying hydrochloric acid by 2.
- Double Displacement:
Consider lead nitrate reacting with potassium iodide:
Pb(NO3)2 + 2KI → PbI2 + 2KNO3
🔗 Note: The nitrate and iodide ions switch places while maintaining balance.
- Combustion:
When methane burns in oxygen:
CH4 + 2O2 → CO2 + 2H2O
🔗 Note: The coefficient of oxygen reflects the stoichiometric requirement for complete combustion.
Applying Balancing Techniques
Balancing chemical equations involves trial and error as well as knowledge of reaction types. Here are some tips:
- Start by balancing elements that appear in only one reactant and one product.
- Leave polyatomic ions in their group if they remain unchanged.
- Use the method of 'least common multiples' for elements in multiple places.
Practical Examples

| Reaction Type | Reactants | Balanced Products |
|---|---|---|
| Combination | Na + Cl2 | 2NaCl |
| Decomposition | KClO3 | 2KCl + 3O2 |
| Single Displacement | CuSO4 + Fe | FeSO4 + Cu |
| Double Displacement | AgNO3 + NaCl | AgCl + NaNO3 |
| Combustion | C3H8 + O2 | 3CO2 + 4H2O |

By following these examples and understanding the basic principles, balancing chemical equations becomes an exercise in pattern recognition and logical deduction.
Tying it All Together

In conclusion, understanding the different types of chemical reactions is essential for mastering the art of balancing chemical equations. Each reaction type has its own set of rules and characteristics, which, once learned, can make balancing more intuitive. These skills are crucial for stoichiometry, predicting reaction outcomes, and understanding the conservation of mass in chemistry.
Remember, practice is key. By working through various examples, you’ll not only remember the reaction types but also become adept at balancing equations swiftly and accurately. The ability to balance equations efficiently not only helps in academic settings but is also fundamental in real-world applications, such as industrial chemistry, environmental science, and biological systems.
What is the simplest way to balance chemical equations?

+
The simplest way to balance chemical equations involves following these steps: Identify the reactants and products, count the number of atoms on each side, start by balancing elements that appear in only one compound on each side, use coefficients to balance the equation, and finally, double-check your work.
Can an equation be unbalanced in real life?

+
In chemical reactions, the law of conservation of mass dictates that atoms cannot be created or destroyed. However, when considering incomplete reactions or reactions that do not go to completion, an equation might not balance in terms of the amount of substances produced or consumed due to various factors like side reactions or impurities.
Why is it important to balance chemical equations?

+
Balancing chemical equations is crucial because it adheres to the law of conservation of mass, which states that mass cannot be created or destroyed in a chemical reaction. It allows chemists to predict the amount of product that will be produced from a given amount of reactants, ensuring accuracy in chemical synthesis and stoichiometry calculations.



