5 Essential Electron Configuration Tips for Chemistry Success

Mastering electron configuration is crucial for understanding chemistry. It's a foundational concept that impacts how atoms bond, interact, and form compounds. Whether you're tackling chemistry at a high school level or delving into more advanced topics, here are some essential tips to help you succeed in mastering electron configuration.
Understand the Basics
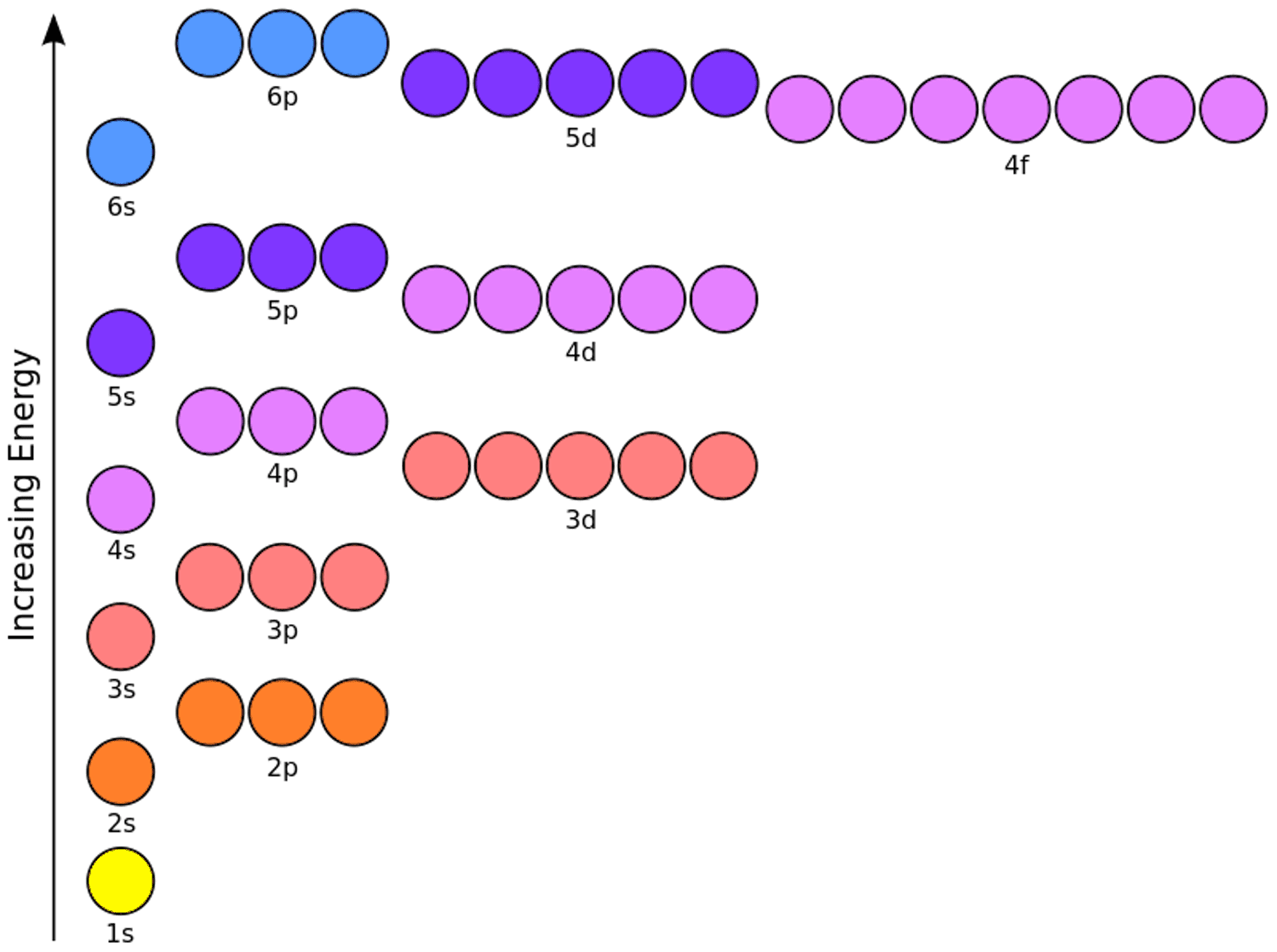
Electron configuration is the way electrons are arranged around an atom’s nucleus. This arrangement is governed by four principles:
- Pauli Exclusion Principle: No two electrons in an atom can have the same set of four quantum numbers.
- Hund’s Rule: Electrons occupy orbitals singly before pairing up.
- Aufbau Principle: Electrons fill orbitals starting from the lowest energy level.
- Heisenberg Uncertainty Principle: The exact position and momentum of an electron cannot be simultaneously known with unlimited precision.
Know the Order of Filling Electron Shells

The order in which electron shells are filled can be tricky to remember. Here’s a useful mnemonic:
| Energy Level | Sublevel | Order of Filling |
|---|---|---|
| 1s | 1s | 1 |
| 2s | 2s | 2 |
| 2p | 2p | 3 |
| 3s | 3s | 4 |
| 3p | 3p | 5 |
| 4s | 4s | 6 |
| 3d | 3d | 7 |
| 4p | 4p | 8 |
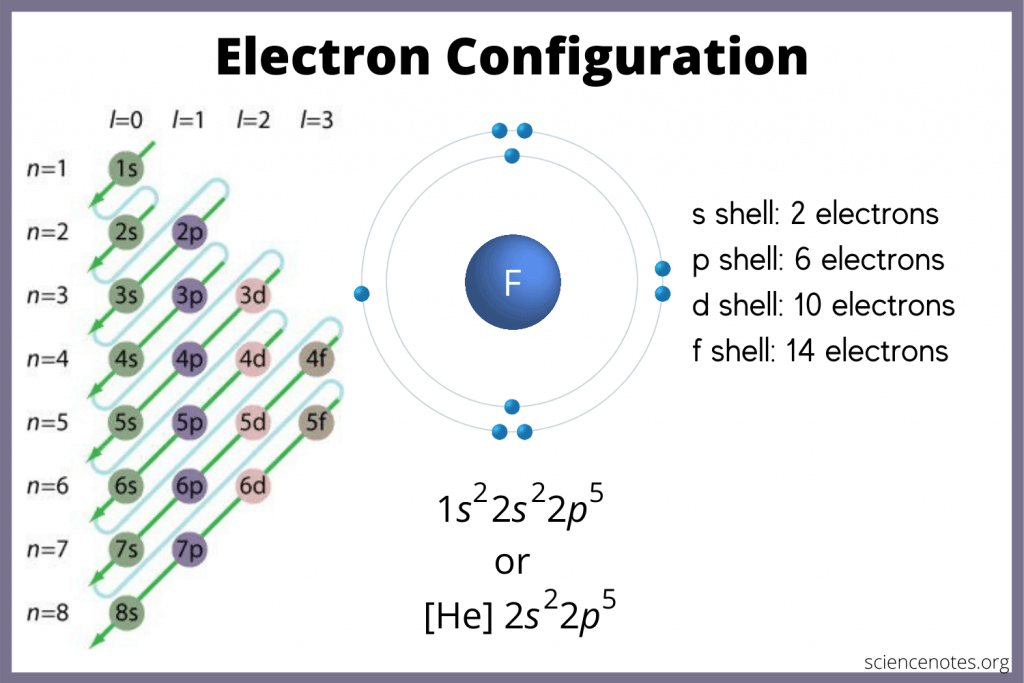
🔍 Note: While this is generally true, there are exceptions where electron configurations deviate from this order for stability reasons, such as in chromium (Cr) and copper (Cu).
Visualize Electron Configurations

Using diagrams can greatly aid in understanding electron configurations:
- Electron Box Diagrams: These show the pairing of electrons in orbitals with up and down arrows.
- Energy Level Diagrams: Show the relative energy levels of electrons.
Visualization helps in reinforcing your understanding of where and how electrons are placed in an atom.
Practice with Different Elements

The best way to get comfortable with electron configurations is through practice:
- Write out electron configurations for elements from different groups and periods in the periodic table.
- Work through the noble gas electron configuration notation, where the configuration is written in terms of the nearest noble gas.
- Use online quizzes or interactive periodic tables to test your knowledge.
Address Exceptions
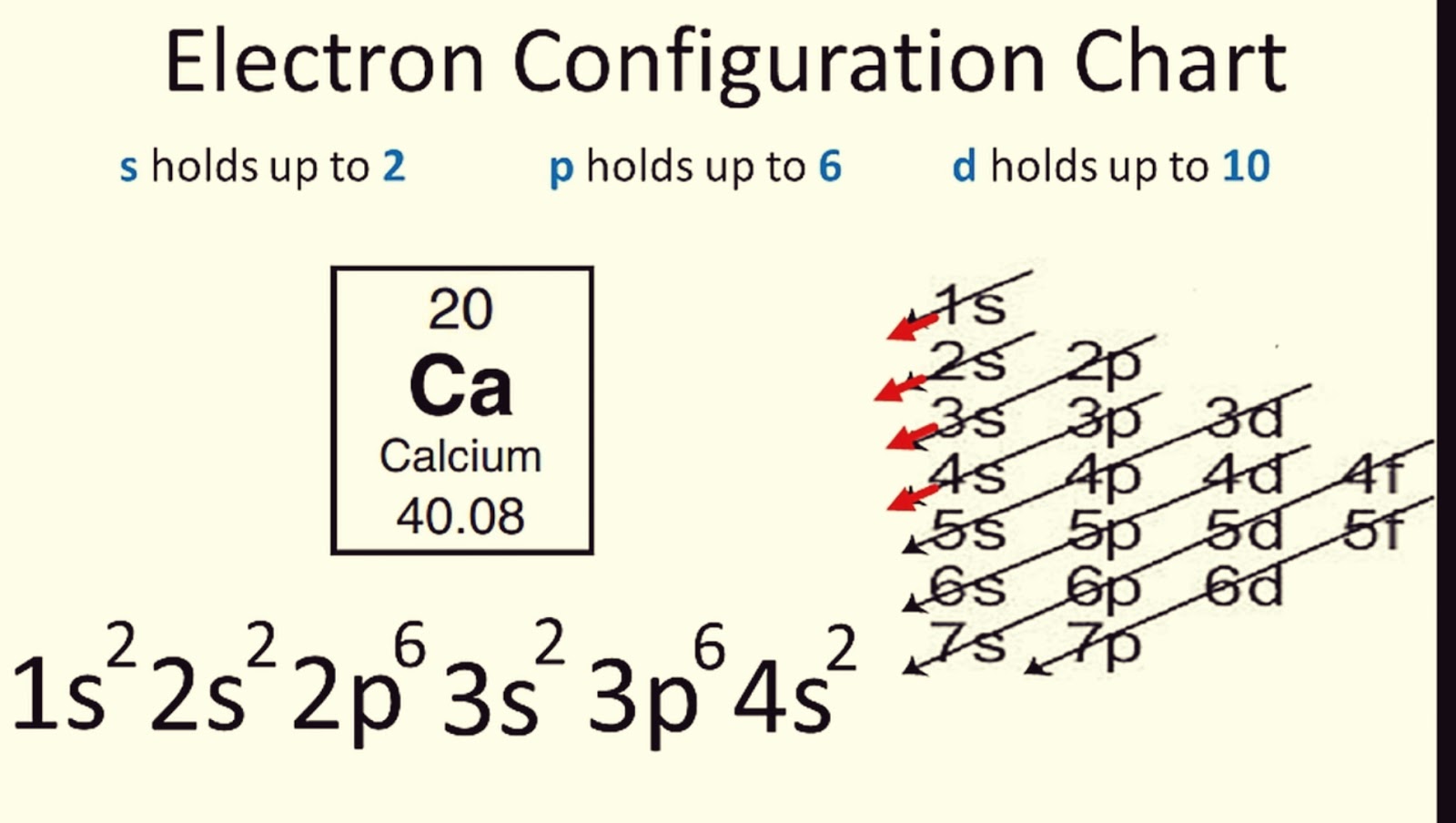
Chemistry often has exceptions, and electron configuration is no different:
- Elements like chromium (Cr) and copper (Cu) exhibit half-filled or fully filled subshells for greater stability, leading to deviations in electron configurations.
Understanding these exceptions involves:
- Recognizing when atoms might break the usual electron configuration rules.
- Studying the electron configurations of elements that are known to have exceptions.
- Considering the concept of electron promotion to gain symmetry or stability in electron distribution.
In summary, electron configuration is not just about remembering the sequence of filling orbitals. It involves understanding the underlying principles that govern electron behavior, using visual aids to grasp the concept better, and recognizing exceptions where chemical intuition and stability play a role. By mastering these tips, you'll not only be able to write out electron configurations with ease but also understand the fundamental reasons behind the atomic structure. This understanding will serve as a robust foundation for further studies in chemistry, from predicting chemical behavior to understanding periodic trends and molecular bonding.
Why is electron configuration important?
+
Electron configuration determines an atom’s chemical properties, its reactivity, and how it will form bonds. It is fundamental to understanding chemical reactions, the periodic table, and the behavior of elements.
What is the difference between a ground state and an excited state?

+
The ground state is the lowest energy level configuration for an electron, where electrons are in their most stable arrangement. An excited state occurs when an electron absorbs energy and jumps to a higher energy level, creating a less stable configuration.
How do electron configurations explain periodic trends?

+
Electron configurations help explain periodic trends like atomic radius, ionization energy, and electron affinity because the arrangement of electrons influences how easily they can be lost or gained, as well as the effective nuclear charge experienced by the outermost electrons.



