Chemistry Unit 1: Size of Things Explained

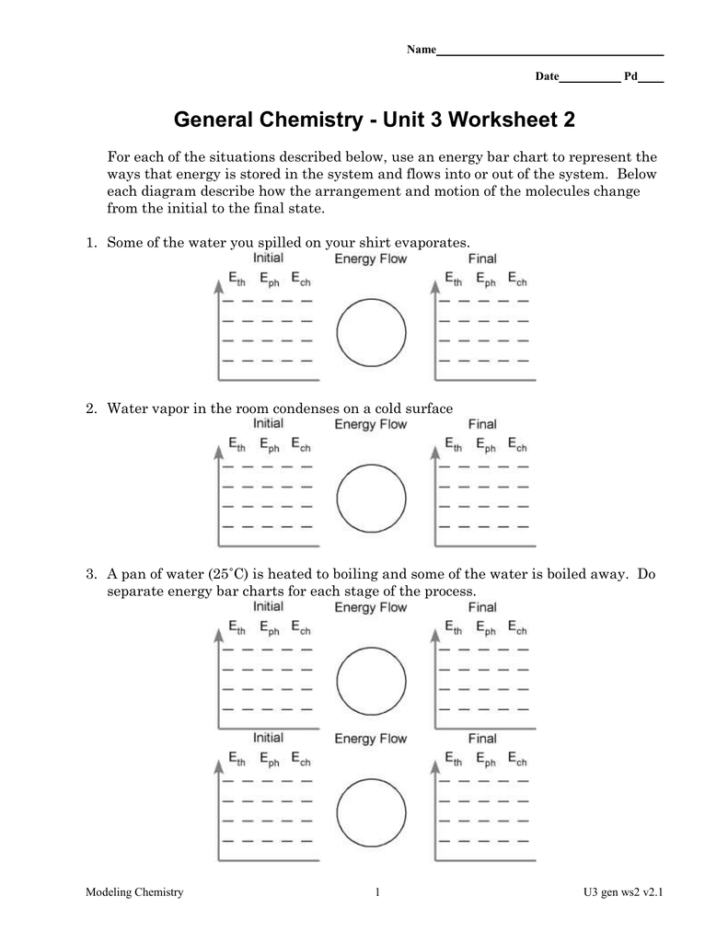
Understanding the Scale: A Dive into the Size of Things
When we look at the world around us, it's often easy to get overwhelmed by the vast array of sizes that exist. From the microscopic intricacies of subatomic particles to the monumental scales of the universe, understanding size is not just about appreciating scale but also about recognizing how this understanding impacts fields like chemistry, physics, and even our daily lives. Let's embark on a journey to understand the science of scale, focusing on chemistry and its units in this first installment.
Subatomic Particles: The Smallest Units
At the very foundation of everything, you'll find subatomic particles. These include:
- Electrons - Orbiting the nucleus, their size is still not defined in classical terms, but they contribute to the atom's overall volume.
- Protons and Neutrons - They make up the atom's nucleus. Protons and neutrons each have a diameter of about 1 femtometer (fm), which is 10^-15 meters.
🧠 Note: The size of subatomic particles is often discussed in terms of probability distributions rather than fixed sizes due to quantum mechanics.
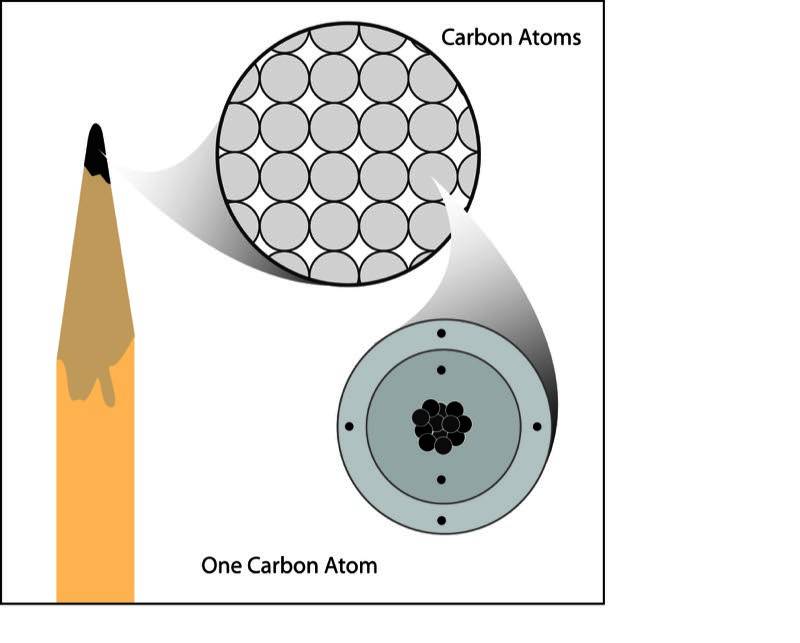
Atoms and Molecules
While atoms are relatively small, with diameters ranging from 0.1 to 0.5 nanometers (nm), they are significantly larger than subatomic particles. Here's what to know:
- An atom's size depends on its atomic number and electron configurations.
- Molecules are larger, their size determined by the sum of their constituent atoms plus any additional volume due to chemical bonds.
Size and Bonding
The size of molecules is directly influenced by the type and number of chemical bonds. For instance:
- A simple diatomic molecule like H2 would measure around 0.74 nm.
- More complex molecules like benzene (C6H6) can extend up to 1.2 nm.
Nanoscale Materials

The nanoscale is where materials exhibit unique properties due to their size. This size ranges from:
- 1 to 100 nm - Here, traditional laws of physics begin to transition into quantum mechanics.
| Material | Size Range (nm) |
|---|---|
| Quantum Dots | 1-10 |
| Nanotubes | 0.8-10 |
| Nanowires | 1-100 |

🔬 Note: Nanomaterials are not just small; they often possess properties that can't be predicted from their bulk counterparts, making them vital for advanced technologies.
Macroscopic Structures

Moving up from the nanoscale, we enter the world of everyday objects and structures:
- Human-scale objects: Their size is more familiar, from millimeters to meters.
- Microscopic to Macroscopic Transitions: For example, a single DNA molecule is microscopic, but when coiled up, it forms visible structures like chromosomes.
Beyond Earth
Our journey in understanding size doesn't end at Earth. We need to consider:
- The Solar System: From a few meters in size (like asteroids) to hundreds of thousands of kilometers in the case of stars like the sun.
- Galaxies and Cosmic Structures: Here, we're dealing with distances in light-years, with galaxies reaching sizes up to 100,000 light-years.
This final point in our exploration highlights the enormity of the universe, a reminder of our small but significant place within it.
In summary, understanding the scale from subatomic particles to the entire cosmos provides context to our scientific inquiries. It's not merely academic; it influences our technological progress, medical advancements, and even our philosophical outlook. By appreciating size in its varied forms, we can better grasp the complex systems at work in the world of chemistry, our daily lives, and the vastness beyond.
Why do we measure the size of atoms in nanometers?

+
Nanometers provide a scale that’s appropriate for atoms, balancing between resolution and practicality. It’s large enough to offer a tangible size yet small enough to accurately represent the atomic scale.
How does size influence the properties of materials?

+
At the nanoscale, materials exhibit different properties due to quantum effects and high surface-area-to-volume ratios. These changes can lead to unique chemical reactivity, electrical conductivity, or optical properties not seen in bulk materials.
What role does scale play in astronomical observations?
+
Scale is crucial for interpreting astronomical phenomena. The vast distances involved mean that light travel time can be used to study the past. Similarly, understanding the scale of stars, galaxies, and cosmic structures helps us comprehend their formation, lifespan, and potential for habitability.