5 Must-Know Tips for Electron Configuration Practice

Understanding electron configuration is fundamental for students studying chemistry, as it lays the groundwork for a deeper understanding of the periodic table, chemical bonding, and many other chemical properties. Whether you're a beginner trying to grasp the basics or someone looking to refine their knowledge, here are five essential tips for mastering electron configuration practice.
Tip 1: Understand the Aufbau Principle

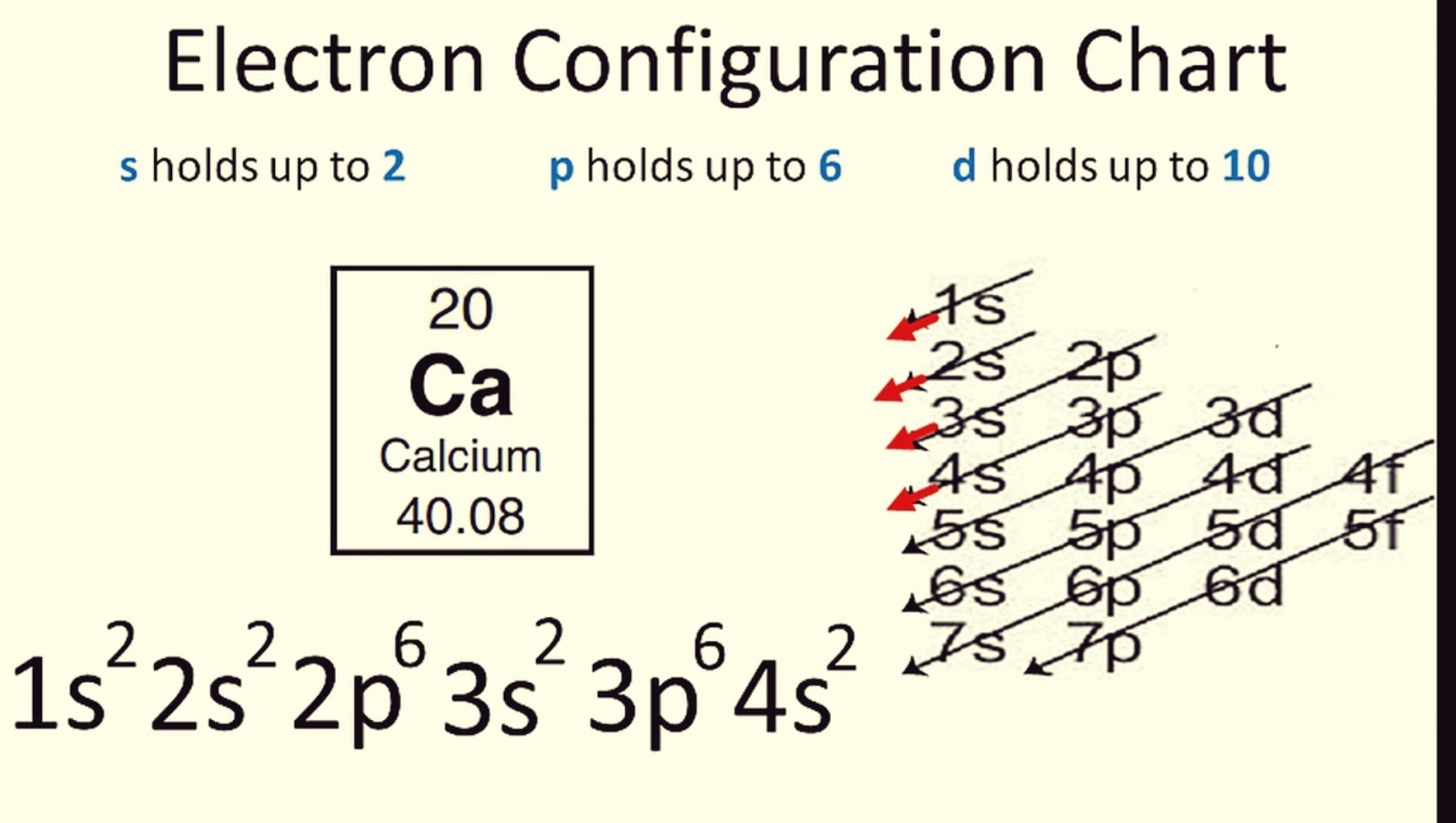
The Aufbau Principle is your starting point in learning electron configurations. It states that electrons fill atomic orbitals of the lowest available energy levels before filling higher ones. Here’s how you can remember the order:
- 1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s
Remembering this sequence can be facilitated through mnemonic devices like the “diagonal rule.” Imagine you’re filling orbitals in a diagonal pattern on the periodic table.
Example: Sodium (Na) has 11 electrons. Its electron configuration would be:
1s² 2s² 2p⁶ 3s¹
Tip 2: Use the Periodic Table as a Reference

The periodic table isn't just a list of elements; it's a map that organizes elements by their electron configurations:
- S-block: Elements in groups 1 and 2 (except hydrogen and helium) where the last electron is in an s-orbital.
- P-block: Includes groups 13-18, with the outermost electrons in p-orbitals.
- D-block: Transition metals where the last electron is in a d-orbital.
- F-block: Lanthanides and actinides where electrons are added to the f-orbitals.
👉 Note: Always count electrons in the correct order, especially when moving from s to d or f orbitals.
Tip 3: Master the Electron Distribution Exceptions
While the Aufbau principle generally works, there are exceptions due to the stability of half-filled and fully filled subshells:
| Element | Predicted Configuration | Actual Configuration |
|---|---|---|
| Chromium (Cr) | [Ar] 3d⁴ 4s² | [Ar] 3d⁵ 4s¹ |
| Copper (Cu) | [Ar] 3d⁹ 4s² | [Ar] 3d¹⁰ 4s¹ |

The reason for these exceptions is that having half-filled or fully filled subshells leads to greater stability.
Tip 4: Use Mnemonics for Electron Configuration

Electron configurations can be complex, but mnemonics can simplify the process:
- Silicon Memory Trick: “Silly Penguins Silly Sailors Paddle Silly Little Boats”
- Periodic Table Trick: Remember ‘Aufbau Diagonal’ for orbital filling order.
👉 Note: Mnemonics are great for quick recall but ensure you understand the underlying principles.
Tip 5: Practice with Diagrams and Visual Tools

Diagrams and visual representations help cement the concept of electron filling:
- Orbital Diagrams: Draw electrons in boxes or circles, following the rules of the Hund’s rule, which states that electrons will occupy degenerate orbitals singly before pairing up.
- Electron Configuration Charts: Use pre-drawn charts where you can fill in the electrons as you learn.
- Apps and Online Tools: There are many apps and online tools where you can practice filling orbitals interactively.

In summarizing, mastering electron configuration is a journey that involves understanding fundamental principles like the Aufbau rule, recognizing exceptions, using memory aids, and practicing with diagrams. With these tips, you'll enhance your proficiency in electron configurations, making chemistry more understandable and intuitive. Keep practicing, and remember that each element's unique electron configuration tells a story of stability and chemical behavior.
Why is the Aufbau Principle important in electron configuration?

+
The Aufbau Principle helps us understand how electrons fill orbitals in an atom, providing a structured way to determine an element’s electron configuration.
Can you explain why some elements have electron configuration exceptions?

+
Some elements like Chromium and Copper exhibit exceptions due to the stability of half-filled or fully filled subshells, which results in lower energy states and greater stability.
How do mnemonics help in learning electron configurations?

+
Mnemonics serve as memory aids, making complex information easier to remember by associating it with simpler phrases or patterns.
What are some good practices for remembering electron configurations?

+
Good practices include regular practice, using visual aids like diagrams, understanding the underlying principles, and leveraging memory aids like mnemonics.