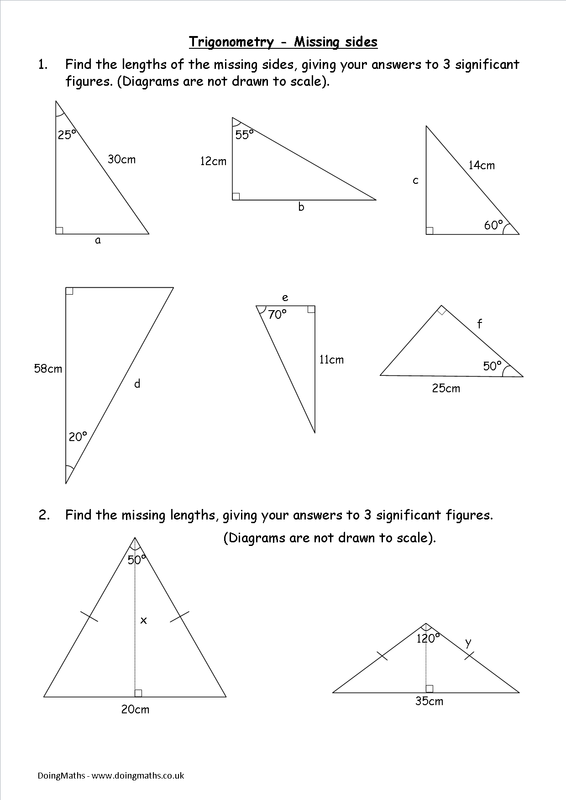
Egg Osmosis Lab Worksheet: Fun Science Experiment

In this exciting experiment, we'll dive into the fascinating world of osmosis using a humble egg. Osmosis is a process where solvent molecules (like water) move through a semipermeable membrane from an area of low solute concentration to an area of high solute concentration, striving to equalize concentrations on both sides. This osmosis egg experiment offers students and science enthusiasts a tangible way to understand this biological and chemical phenomenon. Here's a step-by-step guide on how to conduct the experiment, along with what you'll need and what you can expect to learn.
What You’ll Need

- Eggs (at least two) - White vinegar - Corn syrup or sugar syrup - Salt - Water - Measuring cups and spoons - Four bowls or cups - Food coloring (optional for visualization) - Ruler or tape measure
Procedure

-
Prepare the Eggs


Place the eggs in a bowl or cup filled with white vinegar. Ensure that the eggs are completely submerged. This process removes the eggshell, leaving behind a semipermeable membrane.
📌 Note: Let the eggs sit for 24 to 48 hours. You’ll notice the shell dissolving, leaving the egg membrane soft and rubbery.
-
Rinse and Start the Experiment

After the eggs are de-shelled, gently rinse them with water to remove any vinegar residue. Now, you’re ready to start the osmosis experiment:
- Place one egg in a cup of plain water.
- Place another egg in a cup of corn syrup or sugar syrup.
- Optional: Add food coloring to the water and syrup for better visual effects.
-
Observe and Measure

Wait for 24 to 48 hours, then observe the changes:
- Water Cup: The egg should increase in size as water moves into the egg through osmosis.
- Syrup Cup: The egg will shrink, as water leaves the egg into the higher concentration syrup.
Measure the circumference or volume change in the eggs to document the osmotic movement quantitatively.
📌 Note: Use a tape measure or a ruler for precise measurements. Take pictures for visual documentation.
-
Optional Step with Salt Water

If you want to explore osmosis further, you can place one of the eggs in a concentrated saltwater solution:
- The egg might initially shrink due to the high salt concentration, but if left long enough, it will start to swell as the egg tries to equalize the salt concentration inside and outside.
Understanding the Results

Here’s what you’ve observed through this egg osmosis experiment:
| Solution | Egg’s Reaction |
|---|---|
| Fresh Water | Egg Swells |
| Syrup (High Sugar Solution) | Egg Shrinks |
| Concentrated Salt Solution | Egg Shrinks, then potentially Swells |

This experiment highlights how water moves through semipermeable membranes to balance concentrations. In the water cup, the egg's cytoplasm had a higher concentration of solutes compared to water, causing water to enter. In contrast, the syrup's high concentration of solutes pulled water out of the egg, causing it to shrink. The salt solution creates a similar effect, albeit more complex due to ionic interactions.
The osmosis egg experiment is not just fun; it's educational. It provides a vivid demonstration of osmosis, which is crucial in biology, chemistry, and health sciences. It explains phenomena like why we can't drink seawater, how cells function, and even why saltwater causes dehydration in aquatic organisms.
Why do we use vinegar to remove the eggshell?

+
Vinegar contains acetic acid which reacts with the calcium carbonate in the eggshell, dissolving it and leaving the semipermeable membrane intact.
What happens if we use other liquids like milk or juice?

+
The experiment might still work, but results could vary due to different solute concentrations in milk or juice. For instance, milk might cause similar shrinkage as syrup due to its lactose content.
Can I eat the egg after the experiment?

+
It’s not recommended to eat the egg after the experiment due to potential contamination and the changes in its structure from soaking in various solutions.
Is it possible to reverse the egg shrinking?

+
Yes, by placing the shrunk egg back in fresh water, the egg can reabsorb water and swell up again.