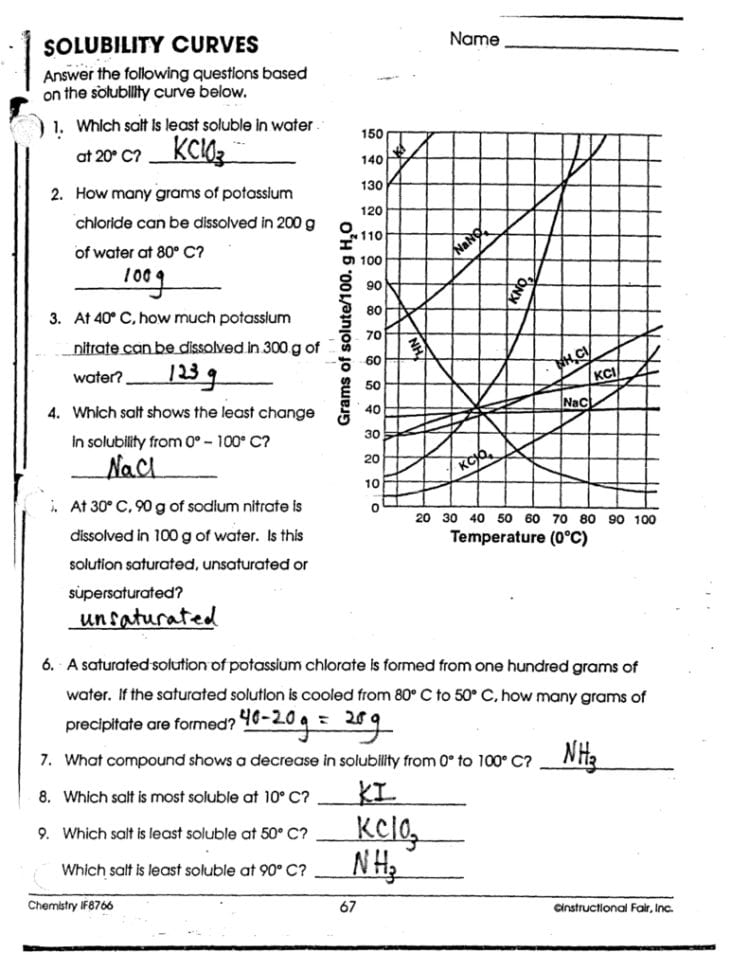
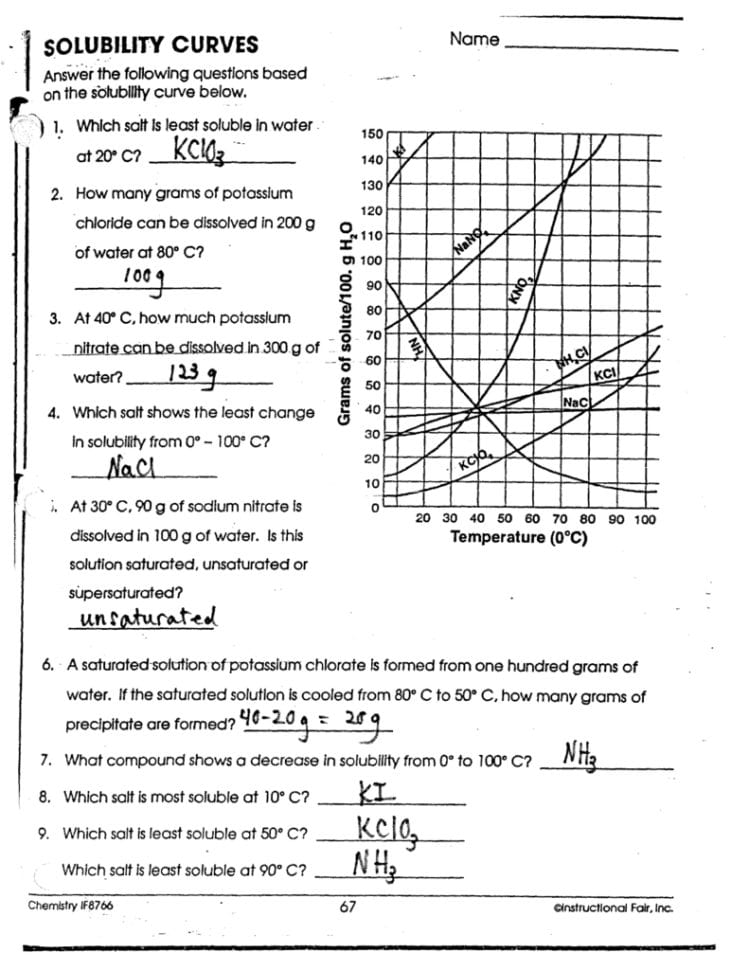
Solubility Graph Worksheet: Answer Key Insights
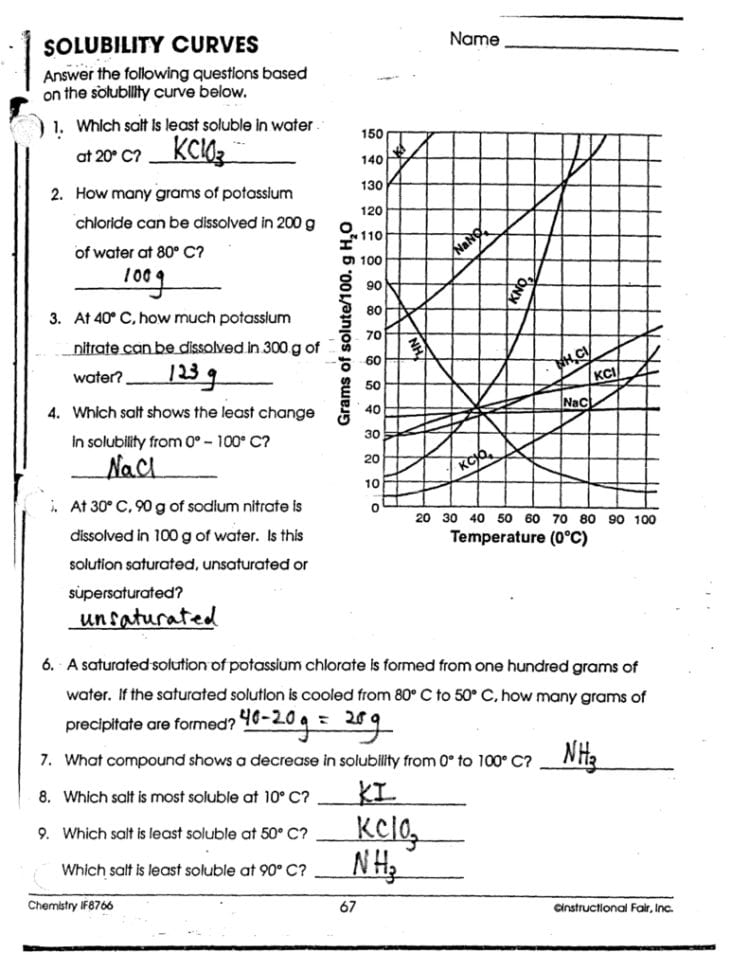
If you're working through a solubility graph worksheet, understanding how to interpret the data can unlock a wealth of information about chemical behavior in solutions. This post will serve as an answer key, guiding you through the key insights and applications of solubility graphs.
Understanding Solubility Graphs
Solubility graphs, also known as solubility curves, visually represent how much solute dissolves in a given solvent at various temperatures. Here are the essential aspects you should understand:
- Axis Explanation: The y-axis typically shows grams of solute per 100 mL of solvent, while the x-axis represents temperature in degrees Celsius.
- Graph Lines: Each line on the graph represents a different solute, allowing for direct comparison across different substances.
Here's a typical example of a solubility graph:

Reading a Solubility Graph
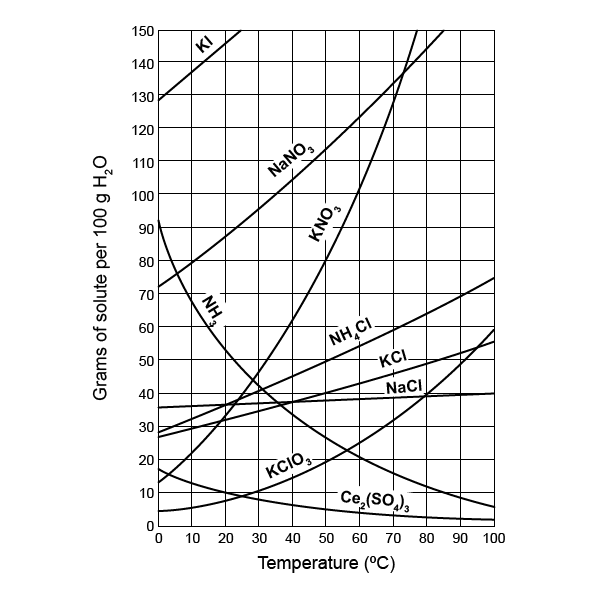
To correctly read a solubility graph, follow these steps:
- Identify the Solute: Find the line that represents the substance you are interested in.
- Locate the Temperature: On the x-axis, find the temperature point you want to analyze.
- Trace Vertically: Draw an imaginary vertical line from this temperature up or down to intersect with the solute's line.
- Read the Solubility: From the point of intersection, draw a horizontal line to the y-axis to read the solubility in grams per 100 mL of solvent.
Understanding this process will allow you to answer most of the questions on a solubility graph worksheet:
📝 Note: Always ensure you're reading the right temperature and line to avoid confusion, especially if multiple substances are on the same graph.
Practical Applications of Solubility Graphs
Here are some practical uses for solubility graphs in various scientific and industrial fields:
- Chemical Engineering: Designing systems that involve crystallization or precipitation reactions.
- Pharmaceuticals: Formulating drug solutions to ensure optimal solubility for medication delivery.
- Environmental Science: Analyzing the solubility of pollutants to predict their behavior in the environment.
- Food Science: Controlling the texture and stability of food products through understanding solubility effects on ingredients.
Analyzing Supersaturation
One interesting aspect of solubility graphs is understanding supersaturation:
- When a solution contains more dissolved solute than can normally be held in solution at a given temperature.
- This can be visually identified when the solubility line exceeds the normal curve for a substance at a given temperature.
Here’s a step-by-step guide to analyze supersaturation:
- Find the Temperature: Determine the current temperature of the solution.
- Compare to Graph: Look at the solubility graph for the solute at that temperature.
- Identify Supersaturation: If the amount of solute in the solution is greater than the maximum solubility, the solution is supersaturated.
💡 Note: Supersaturation is a metastable state; adding a tiny crystal seed or agitating the solution can cause the excess solute to precipitate out.
Creating Your Own Solubility Graph
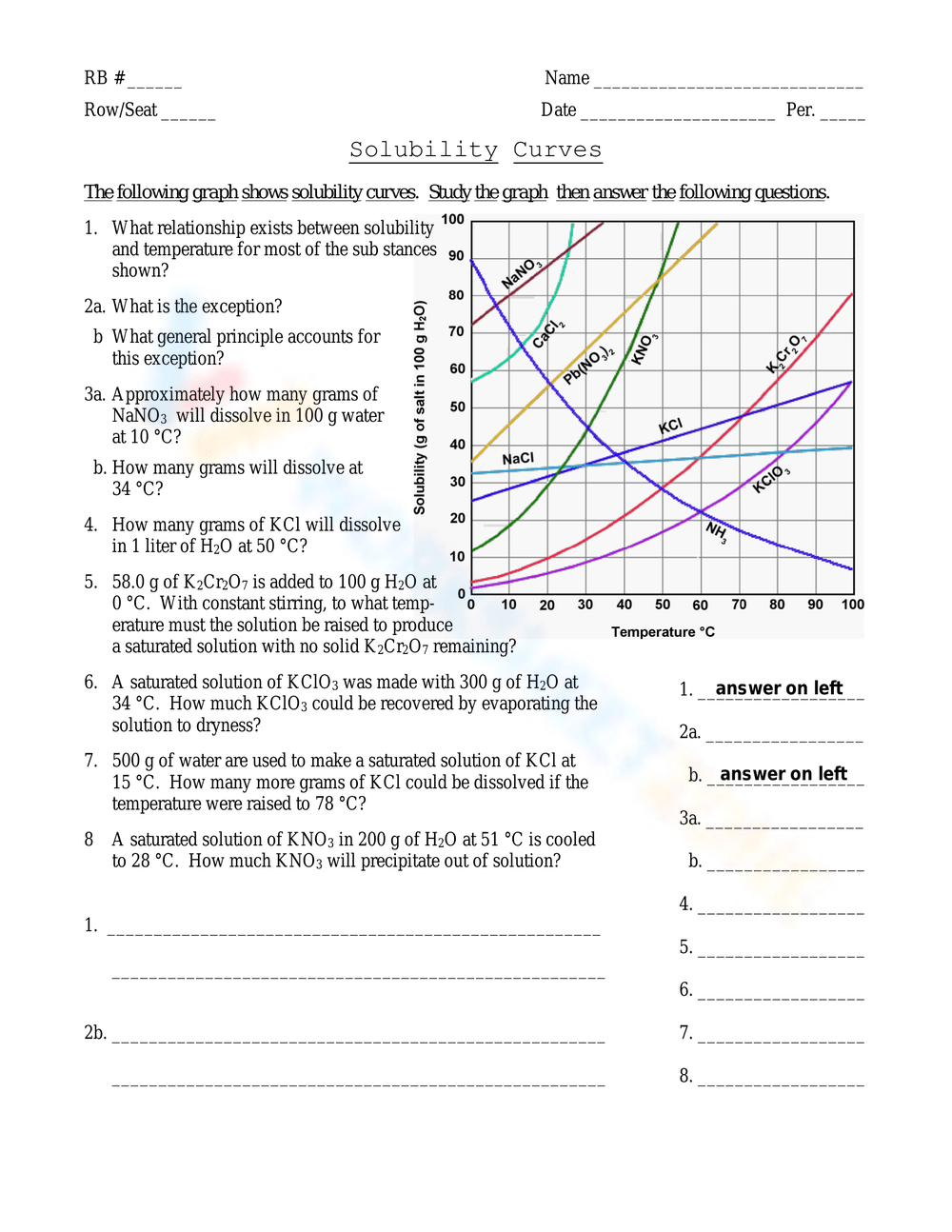
If you’re tasked with plotting your own solubility data, here are some steps to follow:
- Gather Data: Experimentally determine solubility at different temperatures for the solute you’re interested in.
- Plot Points: Mark the solubility values on graph paper, using temperature on the x-axis and solubility on the y-axis.
- Draw the Line: Connect the points to create a smooth curve or line. If the graph is for a mixture, ensure it reflects the combined behavior.
This process not only reinforces your understanding of solubility but also allows for predictive modeling of new compounds or conditions.
📌 Note: When plotting, make sure to scale your axes appropriately to capture the full range of data accurately.
In wrapping up our exploration into solubility graph worksheets, it's clear that these tools provide a visual and analytical bridge between physical chemistry, practical applications, and environmental science. They allow us to predict, control, and understand how substances will behave in solution, offering insights that range from the basic principles of solubility to complex industrial applications. This understanding helps in designing processes, managing industrial applications, and even environmental monitoring, ensuring substances behave as expected when mixed with solvents at varying temperatures.
What is the purpose of a solubility curve?
+
The purpose of a solubility curve is to graphically show how the solubility of a substance in a solvent changes with temperature, allowing for comparisons between different solutes and conditions.
Can solubility graphs predict crystal growth?

+
Yes, by understanding where the solution is relative to the solubility curve, one can predict conditions under which crystallization might occur, especially in scenarios involving supersaturation.
How does the concept of solubility apply in environmental monitoring?

+
Solubility data helps environmental scientists predict how pollutants will disperse and accumulate in water bodies, aiding in pollution control and ecological conservation strategies.