5 Easy Tips for Counting Atoms in Compounds

Understanding the Basics of Atom Counting

Atom counting in chemical compounds is fundamental for students, chemists, and researchers in chemistry. Understanding how to count atoms accurately can help in many areas, from stoichiometry to chemical reactions. Here are five straightforward tips that can help anyone master the art of counting atoms in compounds:
1. Recognize Chemical Symbols and Subscripts

Each element in a compound is represented by a chemical symbol, like "O" for oxygen or "C" for carbon. When atoms appear in multiples within a compound, they are noted with subscripts, like H2O where the "2" signifies two hydrogen atoms. Here are some key points:
- Counting Subscripts: The number following an element symbol indicates how many atoms of that element are in the molecule. For instance, in CO2, there are two oxygen atoms.
- Multiple Elements: If a compound consists of more than one type of element, count each one separately. For example, in glucose (C6H12O6), there are six carbon atoms, twelve hydrogen atoms, and six oxygen atoms.
💡 Note: Always verify that you understand the chemical formula. Incorrect reading can lead to wrong counts.
2. Use Parentheses Correctly

Sometimes, a chemical formula includes parentheses to group atoms or to indicate the presence of polyatomic ions. Here's what to do:
- Multiple Groups: If there's a subscript outside the parentheses, it multiplies the number of atoms inside the parentheses. For example, Ca(OH)2 has two hydroxides, resulting in two oxygen atoms and two hydrogen atoms plus one calcium atom.
- Monatomic vs. Polyatomic Ions: Polyatomic ions, like SO4, are enclosed in parentheses when they appear in multiples within a compound. For instance, Mg(SO4)2 contains two sulfate ions, so two sulfur atoms and eight oxygen atoms.
3. Handle Hydrates with Care

Hydrates are compounds that have water molecules loosely associated with them. This means you'll need to:
- Separate Counts: Count the atoms in the anhydrous (water-free) part of the compound and the water molecules separately. For example, in MgSO4·7H2O, there are one magnesium, one sulfur, and eleven oxygen atoms from the MgSO4 and another 14 from the seven water molecules.
💧 Note: Remember to differentiate between water molecules associated with the compound and those part of the hydrate.
4. Check for Anomalies in Chemical Formulas

Sometimes, compounds have special notation or are part of larger structures:
- Complex Molecules: In biological molecules or large organic compounds, the count can become complex. Here, methodical breakdown into smaller units or parts of the molecule can help in counting accurately.
- Non-stoichiometric Compounds: These compounds do not have a fixed ratio of atoms, like FeO0.94, where iron’s oxidation state varies. Here, consider the average atom counts based on the most common oxidation states.
5. Practice, Practice, Practice
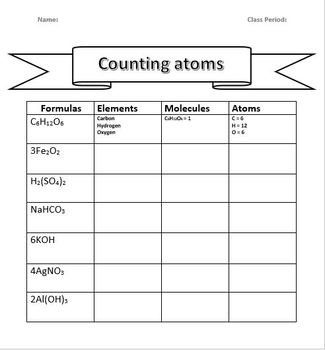
Atom counting, like any skill, improves with practice. Here’s how to refine your skills:
- Work Through Examples: The more you practice with different chemical compounds, the more comfortable you’ll become with different structures and formulas.
- Use Visual Aids: Drawing molecular models or using ball-and-stick models can visually reinforce how atoms are bonded, making counting easier.
- Memorize Common Formulas: Some compounds, like common acids and salts, appear frequently in chemical problems, so knowing their formulas off-hand can save time.
In summary, mastering the art of counting atoms in compounds requires a solid understanding of chemical notation, patience, and consistent practice. Each of these tips helps in breaking down complex formulas into manageable parts, ensuring accurate atom counts, and providing a foundational skill for further study in chemistry.
How do you count atoms in molecules with polyatomic ions?

+
For molecules with polyatomic ions, count the atoms inside the parentheses, then multiply by any subscript outside of the parentheses. For instance, in Na2CO3, there are 2 sodium atoms, one carbon, and three oxygen atoms.
What does the number in front of a chemical formula mean?

+
The number in front of a chemical formula acts as a coefficient, indicating the number of molecules or formula units of that compound present. For example, 2H2O means two water molecules, each containing two hydrogen and one oxygen atom, so in total, there are four hydrogen and two oxygen atoms.
Can you count atoms in an ionic compound differently?

+
Yes, for ionic compounds, you count the atoms based on the formula unit provided. For example, in NaCl, there is one sodium and one chlorine atom, but in a mole of NaCl, there are Avogadro’s number of formula units, so you multiply the atoms count by Avogadro’s number.
What’s the importance of counting atoms in chemistry?

+
Counting atoms is crucial for understanding chemical reactions, balancing equations, determining the stoichiometry of reactions, calculating molar masses, and predicting the behavior of compounds in various chemical processes.
How do you handle counting atoms in molecules with odd numbers in subscripts?

+
When dealing with odd numbers in subscripts, you count them as they are. For instance, in C2H5OH, there are two carbon atoms, six hydrogen atoms (five from the molecule and one from the hydroxyl group), and one oxygen atom.