5 Factors Affecting Enzymes: Worksheet Answers Explained

Enzymes play a pivotal role in biological processes, facilitating reactions that would otherwise occur at a snail's pace. However, their efficacy is significantly influenced by external and internal conditions. Here, we delve into the five crucial factors that affect enzyme activity and provide comprehensive explanations for common worksheet queries associated with these factors.
Temperature
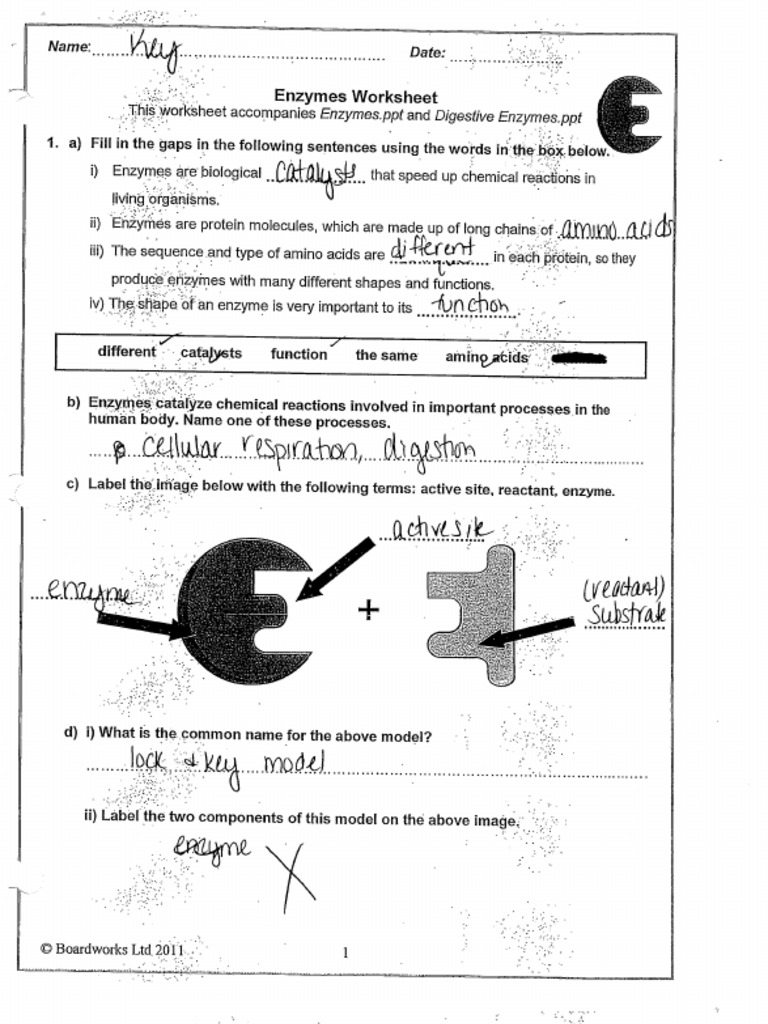
Enzymes are profoundly sensitive to temperature:
- Low Temperatures: At low temperatures, enzyme molecules and their substrates have reduced kinetic energy, resulting in fewer molecular collisions, which leads to a slower reaction rate.
- Optimal Temperature: Each enzyme has an optimal temperature at which its activity is maximized, typically around the organism’s physiological temperature, e.g., 37°C for human enzymes.
- High Temperatures: Beyond their optimal temperature, enzymes undergo thermal denaturation, where their bonds break, leading to a loss of their three-dimensional structure and subsequent loss of function.
Worksheet Questions Explained

- Question: Why does enzyme activity increase with temperature up to a certain point?
- Answer: Enzymes increase activity as temperature rises because this enhances the kinetic energy of both enzyme and substrate molecules, leading to more collisions and more enzyme-substrate complex formations.
- Question: What happens to enzyme activity at extreme high temperatures?
- Answer: Extreme high temperatures cause denaturation, where the enzyme loses its active site structure, leading to a drastic decrease or cessation of enzyme activity.
🔥 Note: Although enzymes denature at high temperatures, some are thermostable, maintaining activity at much higher temperatures than most.
pH

The pH level influences the charge distribution within the enzyme molecule:
- Optimal pH: Enzymes have a specific pH range where they function most efficiently. For example, pepsin, which works in the stomach, has an optimal pH of about 2, while most human enzymes work best at a slightly alkaline pH of around 7.4.
- Outside Optimal pH: Deviations from the optimal pH can alter the charge of amino acids, potentially disrupting ionic bonds, and leading to enzyme denaturation.
Worksheet Questions Explained

- Question: How does pH affect enzyme activity?
- Answer: The pH influences the ionization state of amino acids in the enzyme’s active site. Changes in pH can change the enzyme’s shape and its ability to bind substrates.
- Question: Why do different enzymes have different optimal pH levels?
- Answer: Enzymes work in different environments within the body or ecosystems, and their structure is adapted to function optimally within the pH of those environments.
Substrate Concentration

The concentration of substrate directly impacts the rate of enzymatic reactions:
- Low Substrate Concentration: At low concentrations, the reaction rate increases with substrate concentration as more enzyme molecules can engage with substrate molecules.
- Saturation Point: Beyond a certain concentration, the enzyme becomes saturated, and the reaction rate plateaus because all active sites are engaged.
Worksheet Questions Explained

- Question: What happens to the rate of reaction as substrate concentration increases?
- Answer: Initially, the rate increases with substrate concentration due to more collisions with enzymes. After saturation, the rate does not change significantly since enzymes are fully occupied.
- Question: Why does enzyme activity reach a plateau at high substrate concentrations?
- Answer: At high substrate concentrations, the enzyme is saturated; all its active sites are in use, so adding more substrate does not increase the reaction rate.
Enzyme Concentration

Increasing the concentration of enzymes generally speeds up the reaction rate:
- High Enzyme Concentration: With more enzymes present, more active sites are available, allowing more substrate molecules to be converted to product in a given time.
- Low Enzyme Concentration: If substrate concentration is constant and enzymes are limiting, fewer reactions occur at any given moment.
Worksheet Questions Explained

- Question: How does increasing enzyme concentration affect reaction rate?
- Answer: More enzyme molecules mean more available active sites, which can bind with substrate molecules, thereby increasing the reaction rate.
- Question: What would happen if you doubled the amount of substrate but kept the enzyme concentration constant?
- Answer: Initially, the reaction rate would increase as more substrate would compete for the limited number of active sites. However, once saturation is reached, the rate would not increase further.
📈 Note: In most scenarios, substrate concentration is the rate-limiting factor, not the enzyme concentration.
Inhibitors

Enzyme inhibitors are substances that can reduce or completely block enzyme activity:
- Competitive Inhibitors: Compete with the substrate for the active site, decreasing the effective concentration of the substrate.
- Non-competitive Inhibitors: Bind to an enzyme at a site other than the active site, altering its shape and reducing its effectiveness without competing with the substrate.
- Uncompetitive Inhibitors: These inhibitors bind to the enzyme-substrate complex, stabilizing it, and preventing the formation of products.
Worksheet Questions Explained
- Question: What are the differences between competitive and non-competitive inhibition?
- Answer: Competitive inhibitors vie for the active site, reducing substrate access, while non-competitive inhibitors bind elsewhere, altering the enzyme’s shape, and potentially reducing or stopping activity.
- Question: How can inhibition be overcome?
- Answer: Competitive inhibition can be overcome by increasing substrate concentration, while non-competitive inhibition requires the removal of the inhibitor.
In wrapping up, understanding these five key factors—temperature, pH, substrate concentration, enzyme concentration, and inhibitors—is vital for grasping how enzymes work in biological systems. This knowledge not only helps in answering worksheet questions but also underpins many practical applications in biochemistry and medicine, including understanding drug design, enzyme replacement therapies, and food processing techniques.
How do extreme pH levels affect enzymes?
+
Extreme pH levels can denature enzymes, changing their ionic structure and potentially causing irreversible loss of function.
What’s the difference between reversible and irreversible inhibitors?
+
Reversible inhibitors can dissociate from the enzyme, allowing the enzyme to regain activity, while irreversible inhibitors form a permanent bond, inactivating the enzyme permanently.
Can temperature affect enzymes in cold environments?
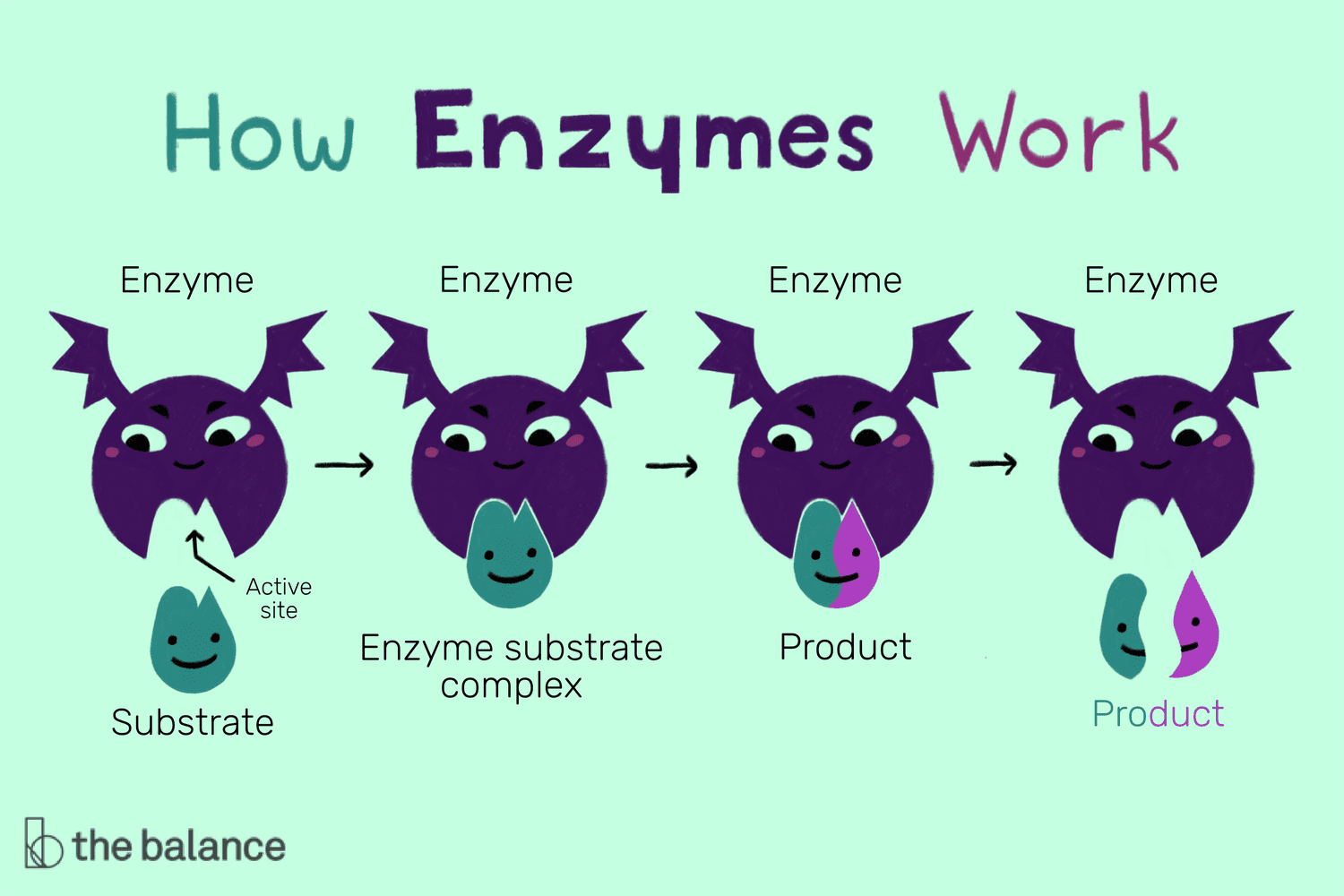
+
Yes, in cold environments, enzyme activity decreases due to reduced kinetic energy, slowing down the rate of enzymatic reactions.
How do enzymes adapt to different conditions?

+
Enzymes adapt through evolution or through regulation mechanisms like allosteric modulation or induction/repression of enzyme synthesis.
Why are some enzymes active at extreme temperatures or pH?

+
These enzymes, often from extremophiles, have evolved unique structural adaptations to maintain stability and function in harsh conditions.