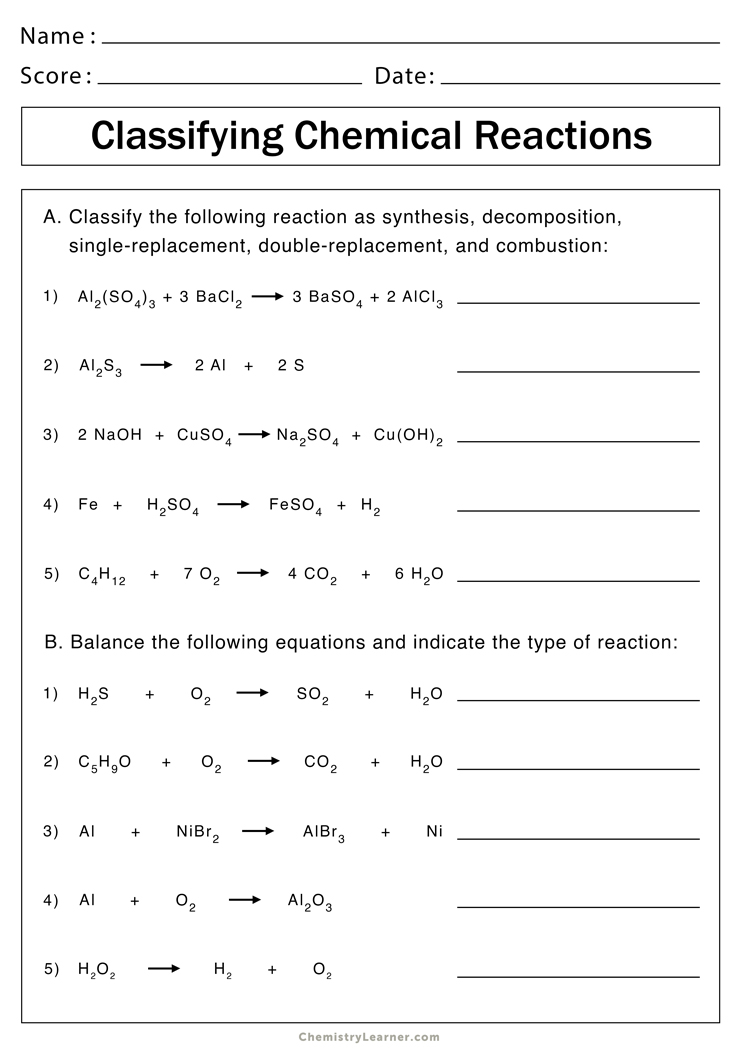
5 Tips for Mastering Reaction Equations in Chemistry Unit 7

Chemistry's reaction equations are foundational to understanding how substances change, interact, and transform, especially in Unit 7 of a typical high school or introductory college chemistry course. This blog post aims to guide students through mastering reaction equations with practical tips to excel in their chemistry studies. Here are five invaluable tips for achieving just that.
1. Understand Stoichiometry

Stoichiometry is the quantitative relationship between reactants and products in a chemical reaction. Here’s how to master it:
- Learn Molar Ratios: Understand how the coefficients in a balanced equation represent the moles of each substance involved. This ratio is crucial for calculating the amount of reactants and products.
- Practice Mass-Mole Conversions: Use the molar mass to convert between grams and moles to understand how much of each substance is needed or produced.
- Limiting Reagent Concept: Recognize the reagent that is consumed first in a reaction, which dictates the amount of product formed.
✏️ Note: Always balance equations before applying stoichiometric calculations to ensure your results are accurate.
2. Familiarize with Common Reactions

Chemistry has several common types of reactions that appear frequently:
- Acid-Base Reactions: Understand neutralization and the formation of salts and water.
- Precipitation Reactions: Learn how to predict when an insoluble compound will form.
- Combustion Reactions: Recognize that these involve the burning of hydrocarbons with oxygen to produce CO2, H2O, and energy.
💡 Note: Each type of reaction has a characteristic pattern, making them easier to identify and balance with practice.
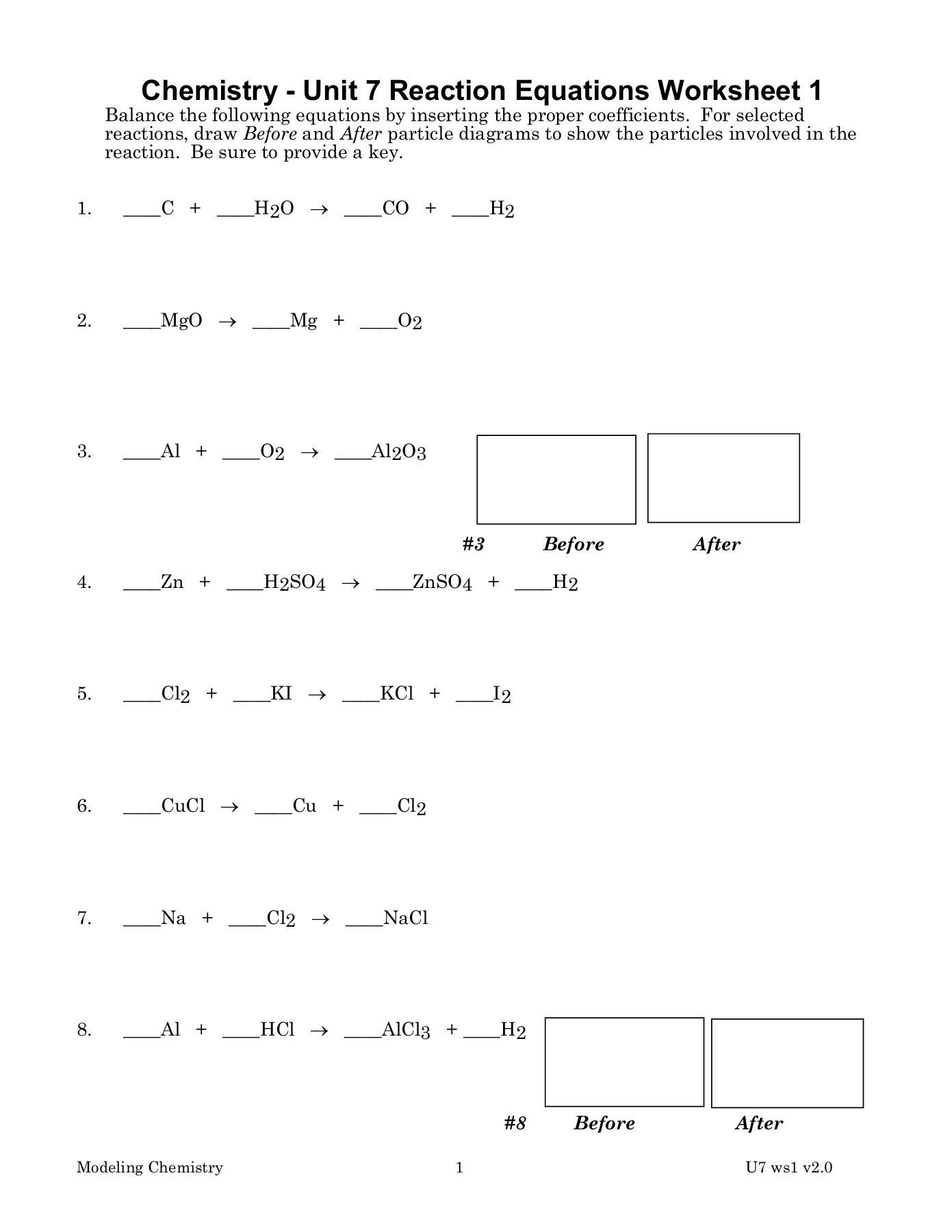
3. Balancing Complex Reactions

Complex reactions can be daunting, but here are strategies to handle them:
- Inspect Method: Start by balancing elements that appear only once on each side, then work on the others.
- Half-Reaction Method: Useful for redox reactions, where you balance atoms and charges separately.
To make complex reactions manageable:
| Reaction Type | Strategy |
|---|---|
| Simple | Direct Balancing |
| Redox | Half-Reaction |
🔍 Note: Don't overlook the oxidation states when dealing with redox reactions as they are key to balancing.
4. Use Limiting Reactants and Excess Reagents
The concept of limiting reactants is essential for solving chemistry problems related to reaction stoichiometry:
- Identify the Limiting Reactant: Use stoichiometry to determine which reactant runs out first.
- Calculate Theoretical Yield: This is the maximum amount of product that can be formed from the limiting reactant.
- Find Excess Reagent: Determine how much of the other reactants remain unreacted.
📊 Note: A clear understanding of limiting reactants helps predict reaction outcomes in real-world scenarios.
5. Continuous Practice and Application

Practice is paramount:
- Solve Various Problems: Each type of reaction has its peculiarities. Solve many problems to get a comprehensive understanding.
- Relate to Real-Life Scenarios: Understand how these reactions occur in the environment or industry.
Chemistry is not merely about knowing the facts but about applying them to understand the world:
- Engage with Experiments: Hands-on experiments help solidify your understanding of reaction equations.
- Study Stoichiometry: Revisit stoichiometry to apply it in various contexts.
Embrace the learning process:
By focusing on these tips, you'll not only become adept at balancing chemical equations but also develop an analytical approach to solving chemistry problems. Remember, Chemistry is an experiential science, so engage with it actively. Keep practicing, ask questions, and seek deeper understanding through real-world applications.
What is stoichiometry, and why is it important?

+
Stoichiometry deals with the quantitative relationships between reactants and products in a chemical reaction. It’s crucial for determining the amounts of substances involved in reactions, understanding reaction mechanisms, and predicting product yields.
How can I improve at balancing complex reactions?

+
Improvement comes with practice. Use the half-reaction method for redox reactions, the inspection method for simpler reactions, and pay attention to the oxidation states to balance charges effectively.
Why are some reactants called “limiting”?
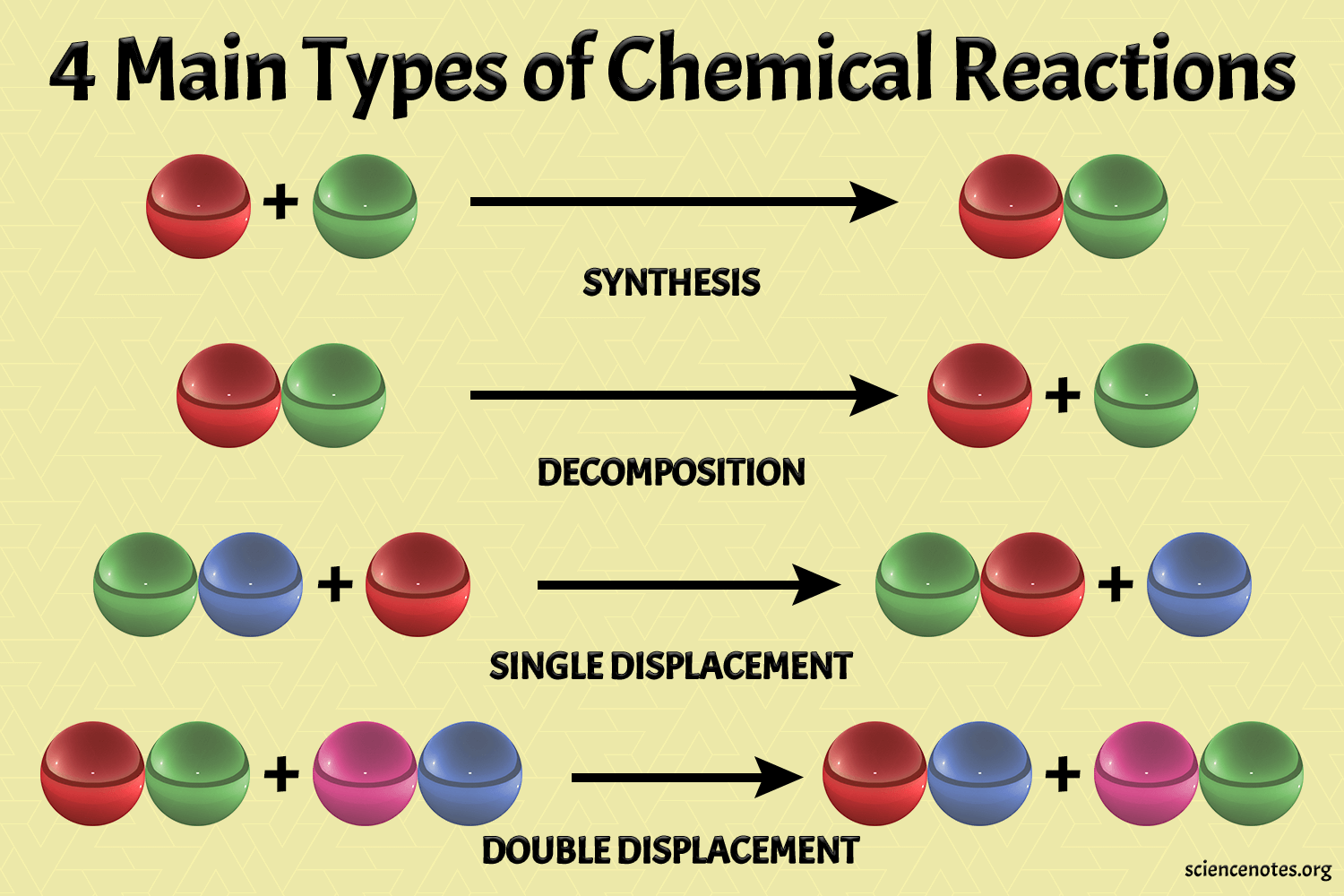
+
A “limiting reactant” is the substance that gets completely used up first, determining the maximum amount of product that can be formed. Other reactants are in excess, meaning not all will react completely.
Can you explain the types of chemical reactions?
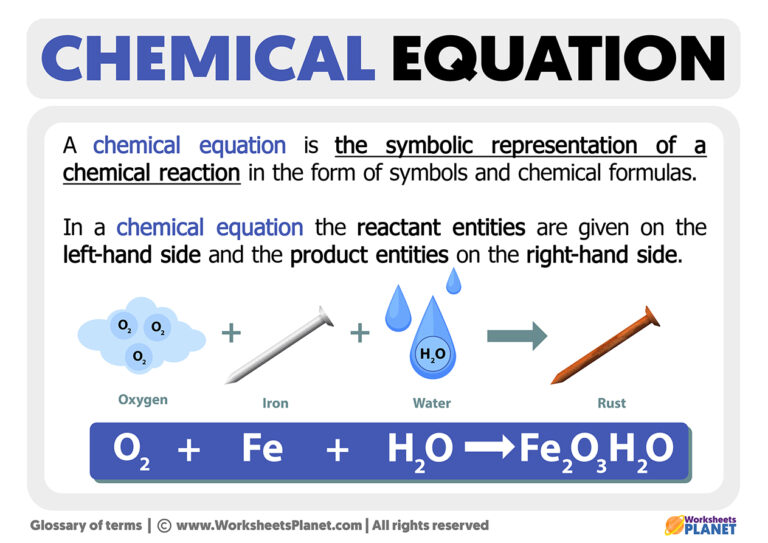
+
Common types include Acid-Base reactions (neutralization), Precipitation reactions (formation of an insoluble solid), Combustion reactions (burning with oxygen), and Redox reactions (electron transfer). Each has distinct characteristics and requires different balancing strategies.
How does practice help in mastering chemistry?

+
Practice helps reinforce theoretical knowledge, improves problem-solving skills, and allows you to apply concepts to different scenarios, ultimately enhancing your understanding and proficiency in chemistry.



