5 Essential Boyle's Law Worksheets for Students

Understanding Boyle's Law is crucial for anyone learning about physics or the sciences that deal with gases. Named after the chemist and physicist Robert Boyle, who first published it in 1662, Boyle's Law states that the pressure and volume of a gas have an inverse relationship when temperature is held constant. This relationship is a foundational concept for understanding gas behavior in various practical and theoretical applications. For students, mastering Boyle's Law can unlock a deeper comprehension of thermodynamics and gas theory.
Why Use Worksheets for Learning Boyle’s Law?

Worksheets are invaluable tools for education for several reasons:
- Hands-On Learning: Students engage directly with the material, enhancing retention.
- Practice: Repetition through exercises helps solidify the understanding of gas laws.
- Assessment: Worksheets offer immediate feedback on students’ grasp of the concept.
- Visual Aid: Tables and diagrams can represent complex ideas in a more accessible format.
1. Boyle’s Law Graphical Analysis Worksheet

This worksheet provides:
- A blank grid to plot pressure vs. volume data.
- Prompts to guide students through the plotting and interpreting of data.
- Questions based on the graphical relationship observed.
Here’s how you can use this worksheet effectively:
- Step-by-Step Analysis: Guide students to analyze data, plot it on the graph, and derive the Boyle’s Law curve.
- Real-World Examples: Use everyday examples to illustrate how pressure and volume relate.
- Discuss Limitations: Encourage discussion on what limitations might apply to the law under different conditions.
📝 Note: Remember that the temperature must remain constant in experiments related to Boyle’s Law.
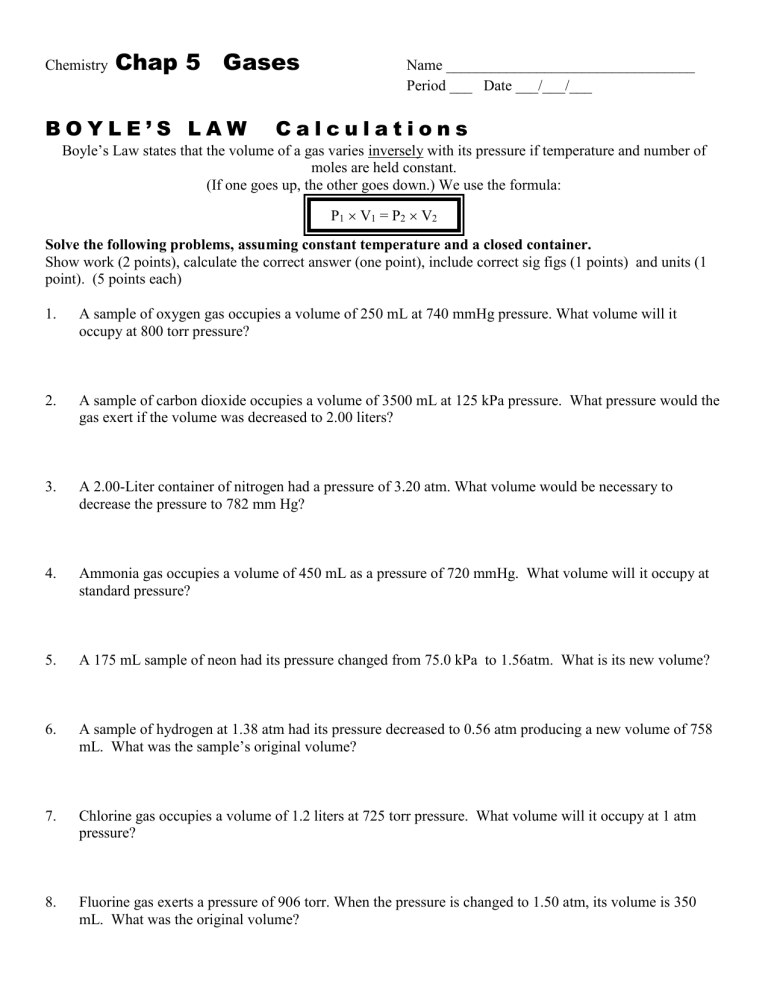
2. Boyle’s Law Problem Solving Worksheet

These worksheets typically contain:
- Mathematical problems involving pressure and volume calculations.
- Scenarios where students need to determine changes in either pressure or volume.
Students will:
- Apply Boyle’s Law formula (P1V1 = P2V2) to solve practical problems.
- Understand the implications of changes in pressure and volume.
- Develop critical thinking skills by evaluating and solving different gas scenarios.
3. Experimental Worksheet

Here, students:
- Perform experiments to demonstrate Boyle’s Law.
- Record data, make measurements, and draw conclusions.
Key aspects include:
- Set Up: Instructions on setting up the experimental apparatus.
- Data Collection: Methods to collect and tabulate data.
- Analysis: Questions to lead students to analyze results in context with Boyle’s Law.
4. Boyle’s Law Applications Worksheet

This worksheet explores:
- Real-life applications of Boyle’s Law in industries like diving, scuba diving, aerospace engineering, etc.
- Problems and scenarios that apply Boyle’s Law to solve engineering challenges.
5. Boyle’s Law Concept Worksheet

Designed to:
- Explain the core concept of Boyle’s Law.
- Provide fundamental exercises to reinforce the understanding of the law.
It often includes:
- Fill-in-the-blank questions to test basic knowledge.
- Short essay questions to articulate the law and its significance.
In wrapping up our discussion on Boyle's Law, it's clear that the journey to mastering this principle involves active learning and practical application. By engaging with these worksheets, students not only commit Boyle's Law to memory but also develop an intuitive sense of how gases behave under varying conditions of pressure and volume. As educators, we encourage the use of these tools to foster a deeper understanding and curiosity in the realm of physical science. The importance of Boyle's Law extends beyond the classroom, affecting everything from how we breath to the design of complex machinery, and thus, its study is both fascinating and foundational.
What is Boyle’s Law?
+
Boyle’s Law states that the pressure of a given mass of gas varies inversely with its volume at constant temperature. Simply put, if you decrease the volume of a gas, the pressure increases, and vice versa.
How can Boyle’s Law be used in real life?

+
Boyle’s Law has numerous applications, such as in diving, where divers experience changes in pressure; in internal combustion engines, where fuel-air mixtures are compressed; and in everyday scenarios like inflating a bicycle tire or balloon.
What are the limitations of Boyle’s Law?

+
Boyle’s Law applies only when the temperature remains constant, which might not be the case in many real-world situations. Also, the law assumes an ideal gas, where intermolecular forces are negligible, which does not always hold true for real gases, especially at high pressures or low temperatures.