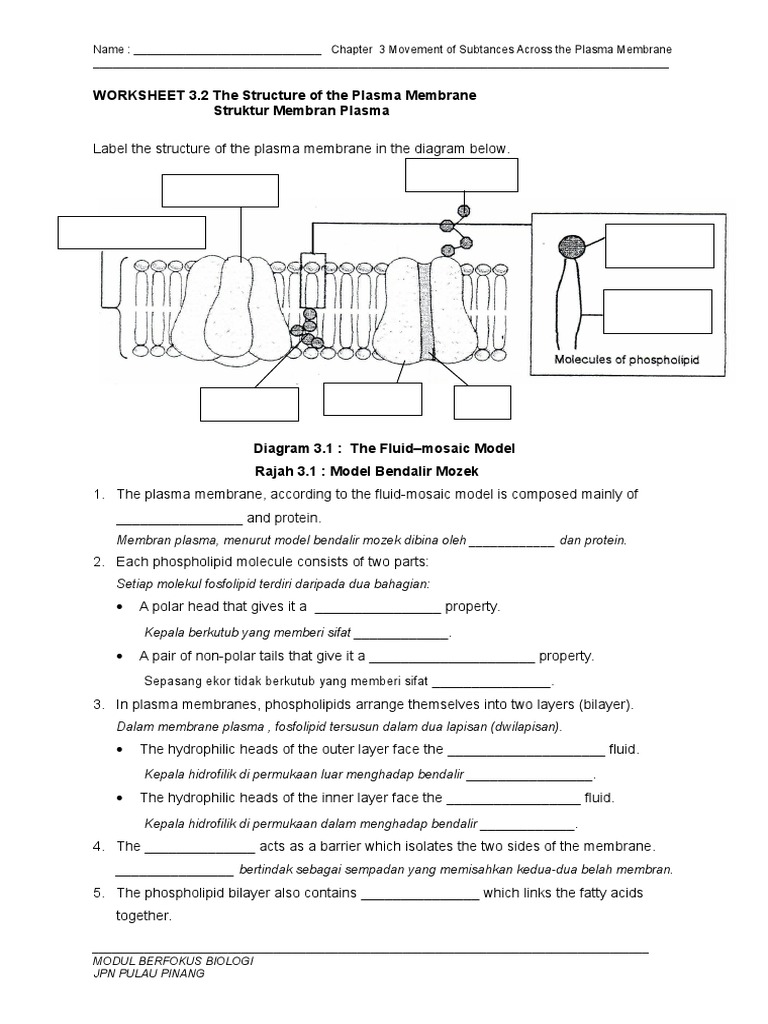
5 Tips for Mastering Bohr Model Practice Worksheets
In the captivating realm of atomic physics, the Bohr model stands as an integral concept to grasp the structure of atoms. This model, crafted by Niels Bohr in 1913, provides a foundational understanding of how electrons orbit the nucleus, using distinct energy levels and fixed orbits. Here are five tips to help you excel in your Bohr model practice worksheets.
Understand the Basics First

Before diving into practice sheets, ensure you have a solid grip on:
- The structure of an atom, comprising protons, neutrons, and electrons.
- Bohr’s theory of quantized energy levels and electron orbits.
- The concept of electron excitation and de-excitation through the emission or absorption of photons.
- The relationships between principal quantum numbers (n), energy levels, and atomic structure.
Understanding these fundamentals will make it easier to tackle more complex problems.
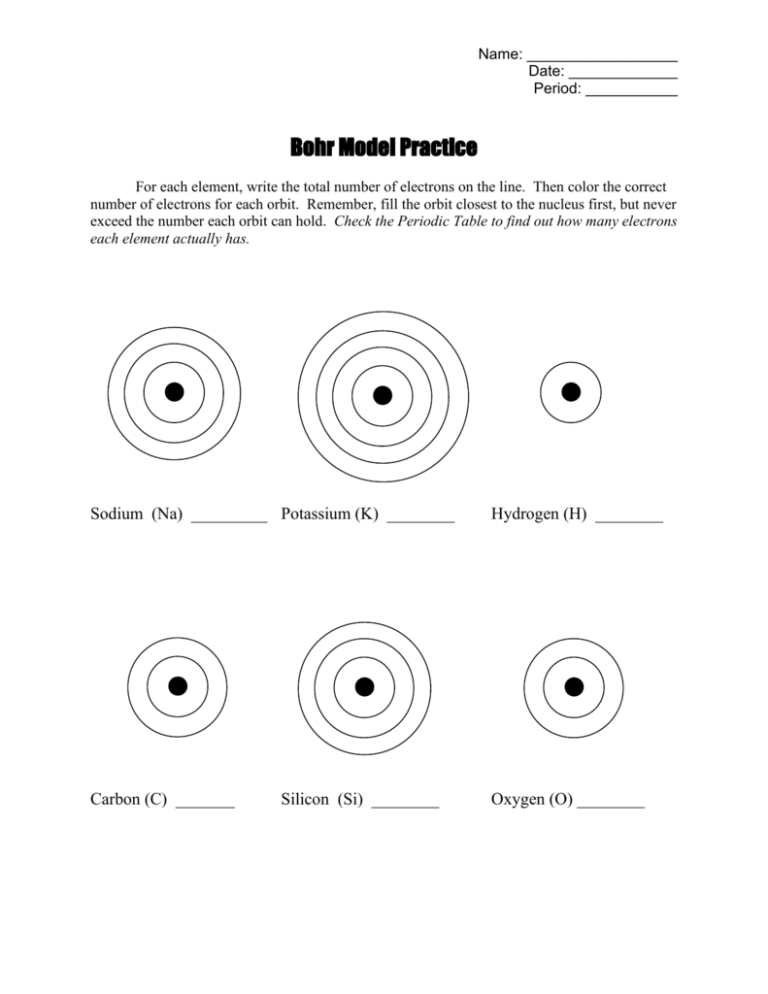
Practice Visualizing


Visualizing atomic models is key:
- Visualize the nucleus as the central mass, with protons and neutrons.
- Picture electron shells or orbits like the layers of an onion.
- Draw simple Bohr models for elements with lower atomic numbers (e.g., Hydrogen, Helium) to build your confidence.
💡 Note: Always use color coding to represent different energy levels for better comprehension.
Master Electron Configuration

| Element | Atomic Number | Electron Configuration |
|---|---|---|
| Hydrogen | 1 | 1s1 |
| Helium | 2 | 1s2 |
| Oxygen | 8 | 1s2 2s2 2p4 |
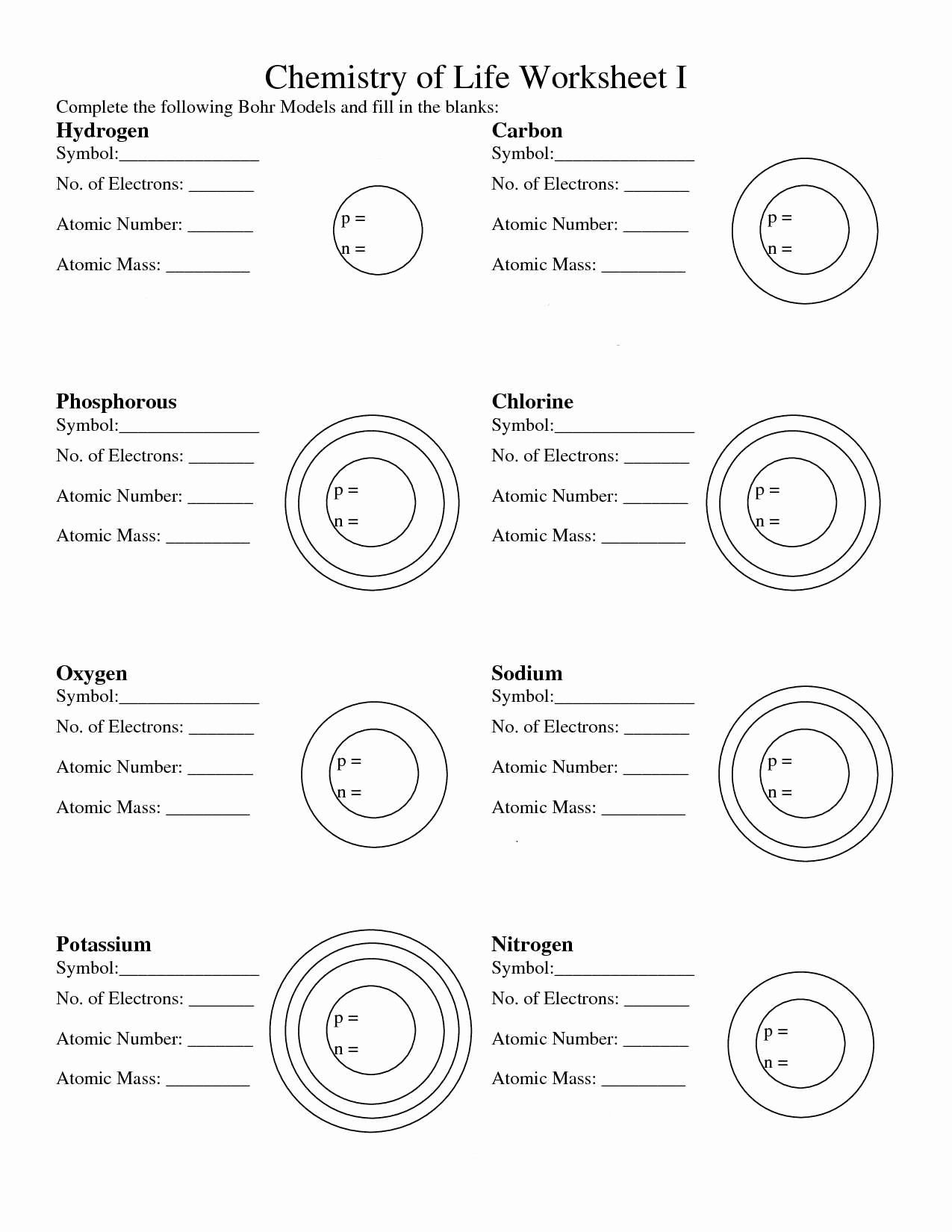
Electron configurations are the key to:
- Determining the valence electrons for elements.
- Understanding how atoms bond.
- Predicting chemical behavior.
Remember, each energy level can only hold a specific number of electrons, dictated by the formula 2n2.
Work with Spectra

When you study the emission and absorption spectra of elements, you’re essentially looking at the fingerprints of atoms:
- Practice linking energy level transitions to spectral lines.
- Understand how different wavelengths correspond to different energy levels.
Here’s a simple yet crucial formula:
E = hc/λ
where E is energy, h is Planck’s constant, c is the speed of light, and λ is the wavelength. Utilize this formula in your practice to find the energy involved in electron transitions.
Analyze Practice Worksheets Thoroughly

When working through your Bohr model practice worksheets:
- Read questions carefully to understand what is being asked.
- Break down complex problems into simpler steps.
- Use diagrams to clarify your understanding.
- Check your work using known data or formulas.
Most worksheets will have:
- Questions to draw Bohr models.
- Questions involving energy calculations.
- Problems on the stability of atoms.
💡 Note: Don’t rush through problems. Take time to grasp each concept and re-evaluate your work for consistency and accuracy.
Summing up, mastering Bohr model practice worksheets entails understanding the core concepts of atomic structure, practicing visualization, mastering electron configurations, working with spectra, and thorough analysis of your work. Keeping these tips in mind will not only help you excel in your atomic physics assignments but also solidify your grasp of atomic behavior, enabling a deeper understanding of chemistry and physics at large.
Why is the Bohr model considered important in atomic theory?
+
The Bohr model provided a breakthrough by introducing the idea of quantized energy levels for electrons, which explained the stability of atoms and the discrete nature of emission and absorption spectra.
How can I determine the number of valence electrons in an element using the Bohr model?

+
Valence electrons are found in the outermost shell of the Bohr model. Count the number of electrons in the highest energy level (n) of the element’s electron configuration.
What are the limitations of the Bohr model?

+
The Bohr model does not fully account for the wave-like nature of electrons, the fine structure of atomic spectra, and fails for more complex atoms due to its inability to explain electron-electron interactions and the need for relativistic quantum mechanics.