Solubility Curve Worksheet 2: 5 Easy Answer Key Tips

In the fascinating world of chemistry, understanding the solubility of different substances is crucial, especially when teaching or learning about solutions and solubility curves. Solubility curve worksheets are often used in educational settings to help students grasp how temperature affects solubility. Here, we'll explore 5 easy tips to help you master Solubility Curve Worksheet 2 with answer keys and provide insights into how to approach these worksheets effectively.
Understanding Solubility Curves


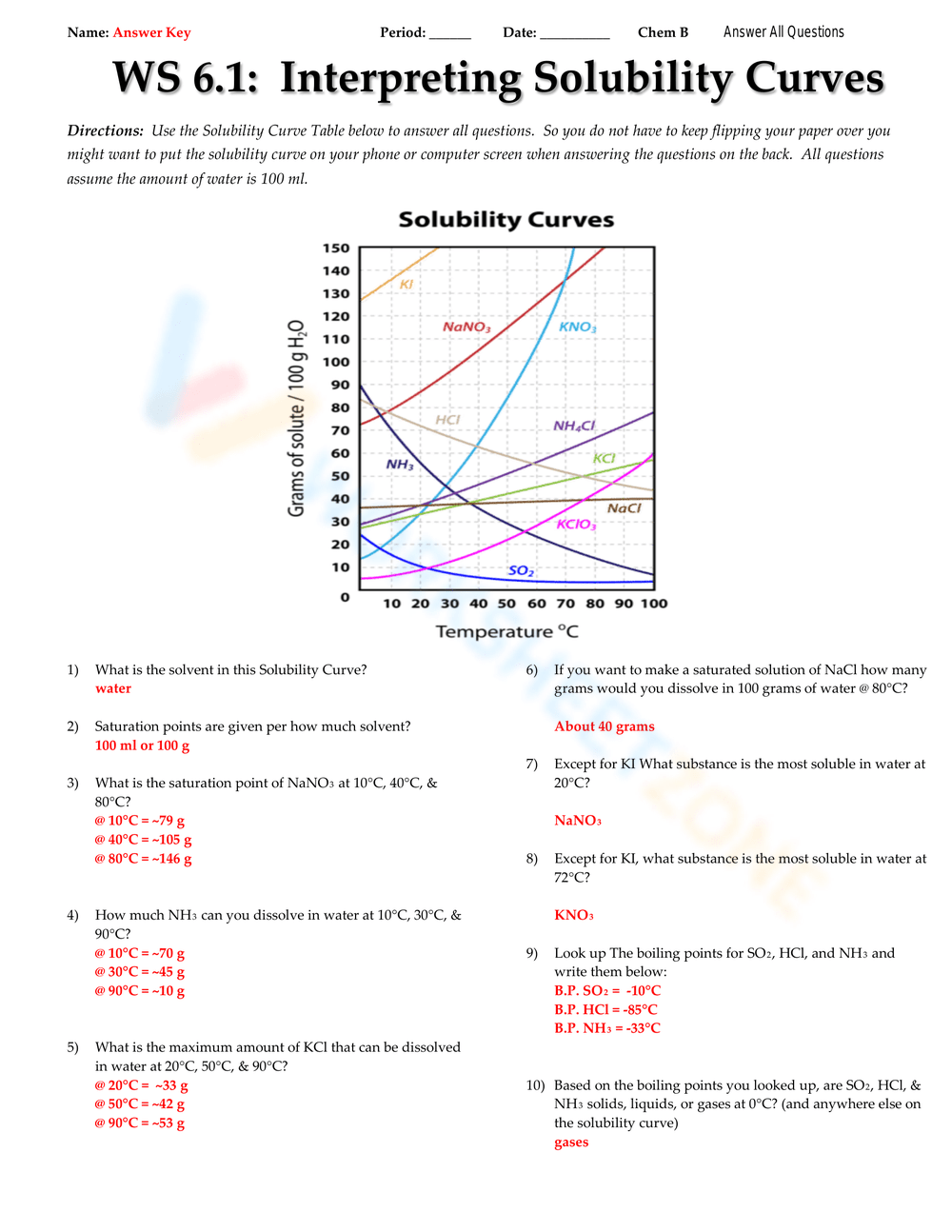
A solubility curve is a graphical representation that shows the amount of solute that can dissolve in a given volume of solvent at different temperatures. Here’s how you can better understand and interpret these curves:
- Identify the Solute: Always note which solute the curve represents. Different solutes will have different solubility behaviors.
- Read the Axis: The x-axis represents temperature, usually in degrees Celsius, while the y-axis shows solubility, often in grams of solute per 100 grams or milliliters of solvent.
- Analyze the Shape: Notice how the curve’s slope changes with temperature. A steeper slope indicates a greater change in solubility per degree of temperature change.
Tip 1: Recognize Patterns
Solubility curves have patterns that can predict solubility behavior:
- Solutes like sodium chloride (NaCl) have a solubility curve that increases with temperature but at a decreasing rate.
- Solutes like cerium sulfate (Ce2(SO4)3) show a sharp increase in solubility at lower temperatures, then level off.
- Some substances might even decrease in solubility at higher temperatures, which is less common.
By recognizing these patterns, you can quickly anticipate how solubility might change with temperature adjustments.
Tip 2: Using Reference Data

Worksheets like Solubility Curve Worksheet 2 will provide you with reference data or solubility curves for various substances:
| Temperature (°C) | Solubility (g/100 mL) |
|---|---|
| 0 | 10 |
| 10 | 20 |
| 20 | 30 |
| 30 | 40 |
| 40 | 50 |

Use this data to plot points or estimate solubility values not directly given.
Tip 3: Interpreting Unsaturated, Saturated, and Supersaturated Solutions

One key aspect of solubility curves is to understand:
- Unsaturated: Below the curve, the solution can still dissolve more solute at that temperature.
- Saturated: On the curve, the solution is in equilibrium with excess solute.
- Supersaturated: Above the curve, the solution contains more dissolved solute than typically possible.
💡 Note: Understanding these states will help in analyzing whether a solution can dissolve more of a substance or if it's already at its solubility limit.
Tip 4: Temperature’s Effect

Temperature plays a pivotal role in solubility:
- For most salts, solubility increases with temperature, but there are exceptions where solubility decreases or remains constant.
- The enthalpy change (ΔH) can be used to predict solubility trends based on the nature of the solute-solvent interaction.
Tip 5: Handling Common Mistakes

Here are common errors students make and how to avoid them:
- Misreading the scale on the graph.
- Confusing mass of solute with concentration.
- Incorrectly assuming that solubility changes linearly with temperature.
By keeping these tips in mind, you can tackle solubility curve worksheets with confidence, ensuring accuracy in your answers and a better understanding of solubility concepts.
To wrap up our exploration of Solubility Curve Worksheet 2, we've covered essential tips that can transform the way you approach these educational tools. Recognizing patterns, using reference data effectively, understanding solution states, considering temperature's effect, and avoiding common mistakes are crucial for mastering these worksheets. This knowledge not only aids in solving problems but also deepens your understanding of chemistry's solubility principles.
What does a solubility curve represent?

+
A solubility curve represents the relationship between the solubility of a solute and the temperature of the solvent. It shows how much solute can dissolve in a given volume of solvent at various temperatures.
How do you know if a solution is unsaturated, saturated, or supersaturated?

+
If a solution is:
- Unsaturated: It can dissolve more solute than it currently holds at a given temperature.
- Saturated: It contains the maximum amount of solute possible at a given temperature, with excess solute present as undissolved.
- Supersaturated: It contains more dissolved solute than normally possible, often achieved by heating and then cooling the solution without disturbing it.
Why does temperature affect solubility?

+
Temperature affects solubility by changing the kinetic energy of the solvent molecules. Higher temperatures increase this energy, allowing for more solute particles to be surrounded by solvent particles, thus increasing solubility. For endothermic processes, solubility might decrease with increasing temperature, as the solute needs heat to dissolve.