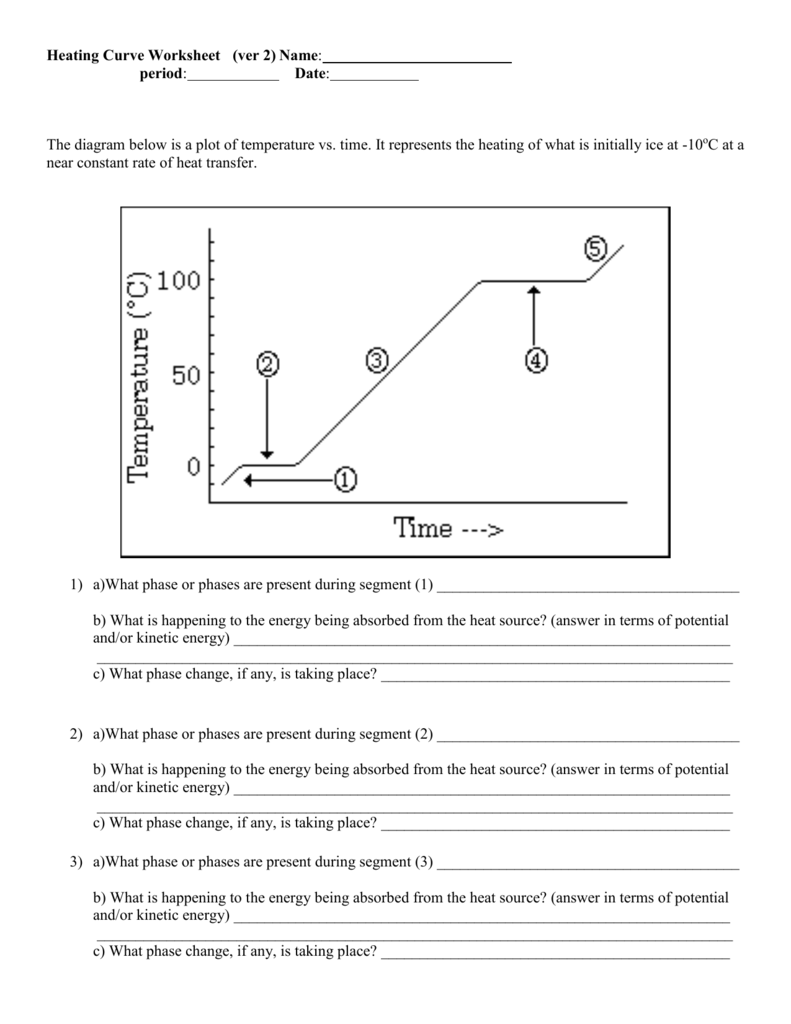
Binary Ionic and Molecular Compounds Worksheet: Side 1 Tips

When diving into the world of chemistry, one often encounters the classification of compounds into two primary types: ionic and molecular. Understanding how to name these compounds correctly is not only fundamental for students but also crucial for chemists working in various fields. Here, we will delve into the methods used to name binary ionic and molecular compounds, which are often covered on the side one of many educational chemistry worksheets.
Understanding Binary Ionic Compounds

Binary ionic compounds consist of a metal cation bonded to a nonmetal anion. The naming convention for these compounds is straightforward:
- The Metal Cation: Simply name the metal element.
- The Nonmetal Anion: Modify the nonmetal's name by changing its ending to -ide. For example, oxygen becomes oxide, sulfur becomes sulfide.
Here are some steps to name binary ionic compounds:
- Identify the metal and write its name as the first part of the compound name.
- Determine the nonmetal and change its ending to -ide.
- Combine the two names, ensuring to keep the metal element's name first.
⚠️ Note: Remember that some transition metals can have multiple charges. In these cases, use Roman numerals to indicate the oxidation state, e.g., Iron(III) chloride for FeCl3.
| Formula | Name |
|---|---|
| NaCl | Sodium chloride |
| MgO | Magnesium oxide |
| AlBr3 | Aluminum bromide |

Naming Binary Molecular Compounds
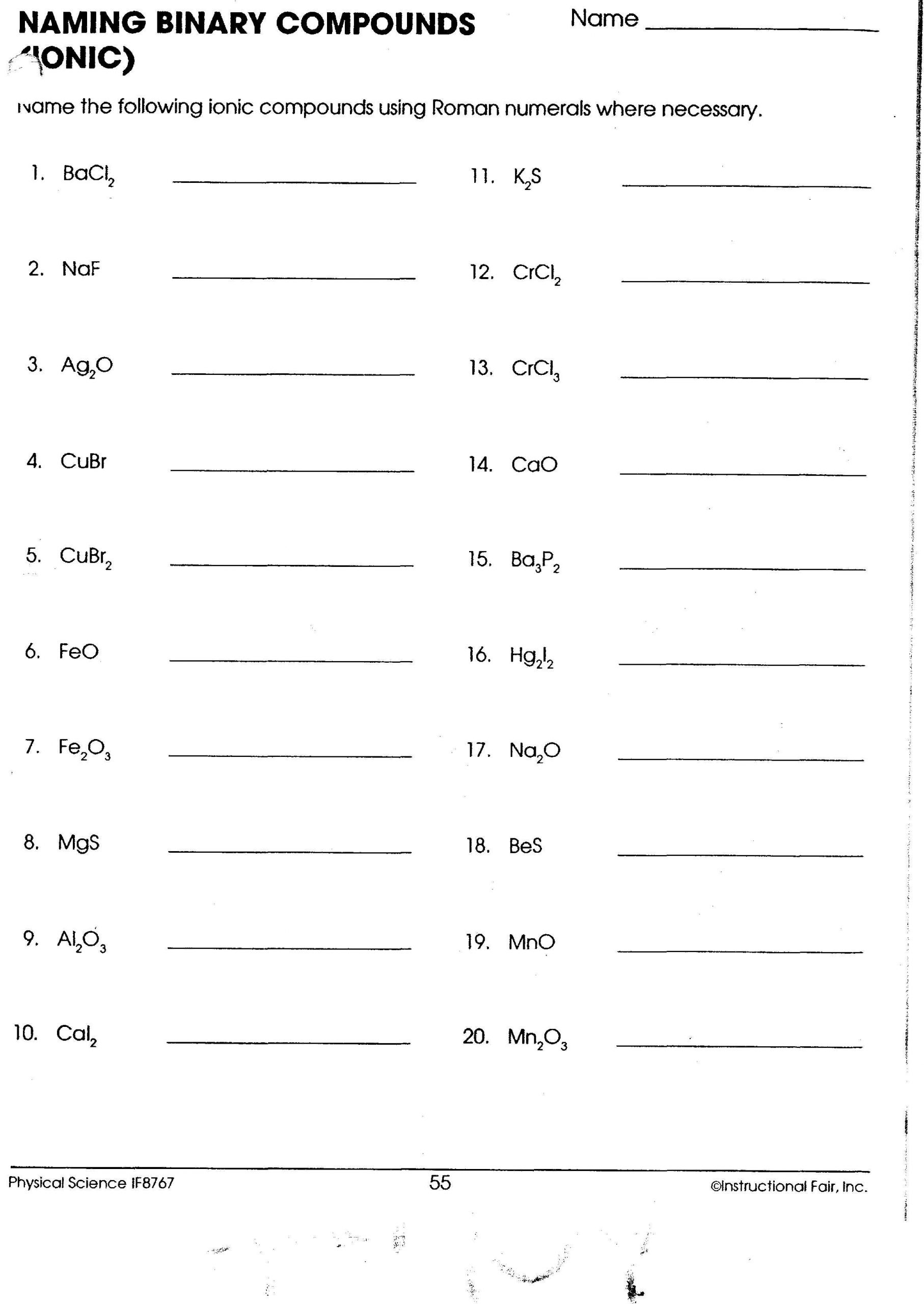
Unlike ionic compounds, molecular compounds involve covalent bonds where atoms share electrons. Here's how to name them:
- Use Greek prefixes to indicate the number of each type of atom present in the molecule.
- Prefixes: mono (1), di (2), tri (3), tetra (4), penta (5), hexa (6), hepta (7), octa (8), nona (9), and deca (10).
- Start with the element less electronegative (generally first in the periodic table).
- Modify the second element with the -ide ending as with ionic compounds.
💡 Note: The prefix "mono" is often omitted for the first element to avoid confusion, but not always. Always check the context or the teacher's guidelines.
| Formula | Name |
|---|---|
| N2O | Dinitrogen monoxide |
| PCl5 | Phosphorus pentachloride |
| CBr4 | Carbon tetrabromide |
Distinguishing Between Ionic and Molecular Compounds

The distinction between ionic and molecular compounds can be made by:
- Checking if the compound is composed of a metal (ionic) or two non-metals (molecular).
- Observing the compound's physical properties; ionic compounds tend to be solids with high melting points, while molecular compounds can be gases, liquids, or low-melting solids.
🔍 Note: The type of bond (ionic vs. covalent) can often be inferred from the electronegativity difference between the elements involved. A difference greater than 1.7 usually suggests ionic bonding.
Practical Tips for Naming

- Practice: Regularly practice naming both types of compounds to improve your familiarity and speed.
- Use Mnemonic Devices: Create mnemonics for remembering Greek prefixes or the names of common ions.
- Understand the Periodic Table: Knowing where elements are located and their properties can help in quickly identifying whether a compound is ionic or molecular.
Applications and Importance
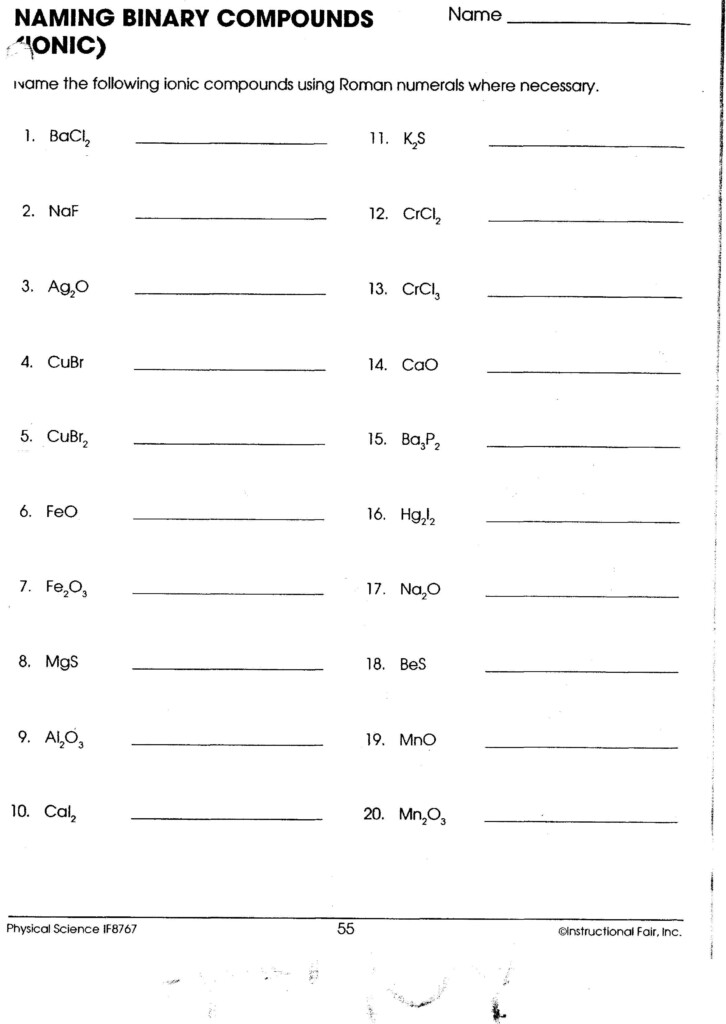
Knowing how to name compounds correctly is vital for:
- Communicating chemical formulas in scientific literature.
- Understanding the composition of substances in industry, from pharmaceuticals to materials science.
- Aiding in the design and synthesis of new compounds in research.
In summary, understanding how to name binary ionic and molecular compounds is crucial for anyone studying or working in chemistry. This knowledge not only allows for clear communication but also provides insight into the properties and reactions of these substances. Through practice and a solid grasp of chemical nomenclature, one can confidently tackle the naming and understanding of a wide variety of chemical compounds.
What is the difference between an ionic and a molecular compound?

+
The primary difference is the type of bonding. Ionic compounds have electrostatic forces holding the ions together, where metal cations and nonmetal anions are bonded. Molecular compounds are formed by covalent bonds where atoms share electrons between nonmetals.
How do you know when to use Greek prefixes?

+
Greek prefixes are used for molecular compounds to indicate the number of atoms of each element in the molecule. They are not used for ionic compounds unless specifying polyatomic ions or when a metal has multiple possible charges.
Can molecular compounds be solids?

+
Yes, while many molecular compounds are gases or liquids at room temperature, some can form solids, especially if they have a high molecular weight or strong intermolecular forces, like in the case of sugar (sucrose).