5 Essential Answers for Heating Curve Worksheet Success
Understanding how substances change phase is fundamental in chemistry, especially when dealing with temperature and energy. A heating curve worksheet is an effective tool to learn these transformations visually and quantitatively. Here, we'll delve into five essential answers that you need to know to ace your heating curve worksheet, providing both foundational knowledge and tips for navigating the worksheet with confidence.
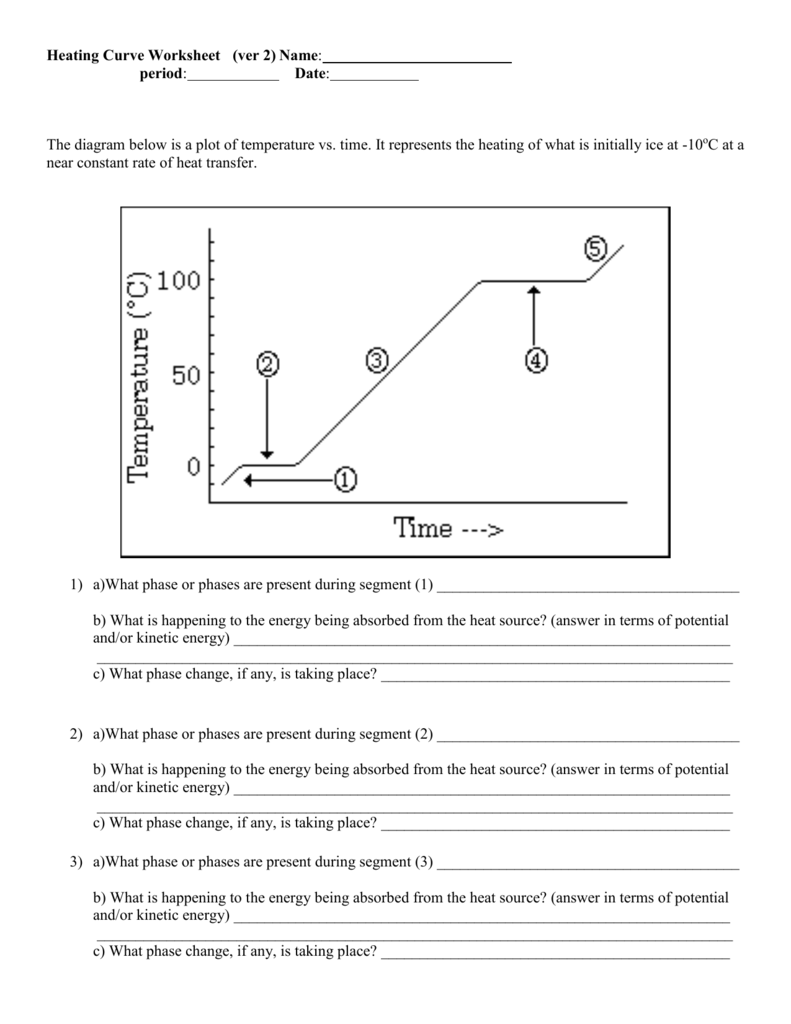
What is a Heating Curve?


A heating curve illustrates the change in temperature of a substance as it is heated. It’s a graphical representation where:
- The horizontal axis denotes time.
- The vertical axis shows the temperature.
The curve itself shows how temperature varies with time, revealing the different phases of matter and phase transitions:
- Solid Phase: The substance starts as a solid, where temperature rises linearly with heat addition.
- Melting: At the melting point, temperature stays constant as the heat added is used to break intermolecular bonds, not to raise the temperature.
- Liquid Phase: After melting, the temperature of the liquid increases as more heat is added.
- Boiling: At the boiling point, the temperature remains constant while the heat energy is used to overcome molecular forces and convert the liquid into gas.
- Gaseous Phase: Finally, the gas temperature rises with continued heating.
🔬 Note: The slope of the curve during heating phases is proportional to the specific heat capacity of the substance.
How to Identify Phase Changes

Phase changes are the flat lines on a heating curve:
- Melting (Solid to Liquid): This occurs when the temperature is at the substance’s melting point.
- Boiling (Liquid to Gas): This happens when the temperature reaches the boiling point.
Here’s how to identify them:
- Look for constant temperature intervals where the heat added causes phase change rather than temperature rise.
- During these periods, the substance is undergoing a latent heat transfer.
Calculating Heat Added During Phase Changes

To find out how much heat is needed to cause a phase change, use the formula:
- Q = m × ΔH
Where:
- m is the mass of the substance.
- ΔH is the heat of fusion or heat of vaporization, depending on the phase change.
💡 Note: The heat of fusion is for melting/freezing, and the heat of vaporization is for boiling/condensation.
Determining Temperature Changes

To calculate the temperature change within a phase:
- Q = m × c × ΔT
Where:
- m is the mass of the substance.
- c is the specific heat capacity.
- ΔT is the change in temperature.
Here are some tips:
- Use the correct specific heat capacity value for the phase of the substance.
- Be careful with units; ensure all values are in the same unit (often joules, J, or calories, cal).
✏️ Note: Different phases of a substance can have different specific heat capacities.
Worksheet Troubleshooting and Common Mistakes

Here are some common issues and tips for working through heating curve problems:
- Not using the right values for latent heat: Ensure you use the correct values for your substance’s heat of fusion and vaporization.
- Confusing specific heat capacities: Remember the phase-specific heat capacities.
- Neglecting units: Always include and convert units properly.
- Missing the enthalpy of fusion or vaporization: Heat is still added during phase changes even though the temperature stays constant.
By mastering these five essential aspects of heating curves, you're not just preparing for a worksheet; you're gaining a deeper understanding of how matter behaves at the molecular level. This knowledge extends to real-world applications like heating systems, industrial processes, and even climate science. Always remember to keep track of units, use the right formulas for each step, and understand the underlying principles of energy transfer.
What if the substance on my worksheet has multiple melting or boiling points?

+
Substances with impurities or mixtures might exhibit a range of melting or boiling points rather than a single value. The heating curve will show a longer flat line for these changes.
Can I use heating curves for cooling processes?

+
Yes, cooling curves are essentially heating curves read backwards. As you remove heat, the temperature will decrease until it reaches phase transition points, where the temperature will hold steady.
How do I handle a substance with variable specific heat capacities?

+
Calculate the heat change for each segment of the heating curve where the specific heat capacity changes, then sum these to get the total heat added or removed.