Nuclear Reaction Balancing Worksheet Answers Unveiled

In the intricate realm of chemistry, nuclear reactions hold a pivotal position. Balancing nuclear reactions, much like balancing chemical equations, involves ensuring that the total mass and charge are conserved. This blog delves deep into the nuances of nuclear reaction balancing, offering comprehensive answers and insights to enrich your understanding.
Understanding Nuclear Reactions

Nuclear reactions involve changes in the nucleus of an atom, resulting in the transformation of elements or isotopes. Unlike chemical reactions, which deal with the rearrangement of electrons, nuclear reactions involve alterations in protons, neutrons, or both, leading to the emission of particles or energy.
Types of Nuclear Reactions

- Fission: The nucleus splits into two or more lighter nuclei, often with the release of a significant amount of energy.
- Fusion: Two light nuclei combine to form a heavier nucleus, accompanied by a release of energy.
- Transmutation: One element is converted into another via bombardment with particles.
- Decay: Radioactive decay where an unstable nucleus emits particles or photons to achieve stability.
The Balancing Act

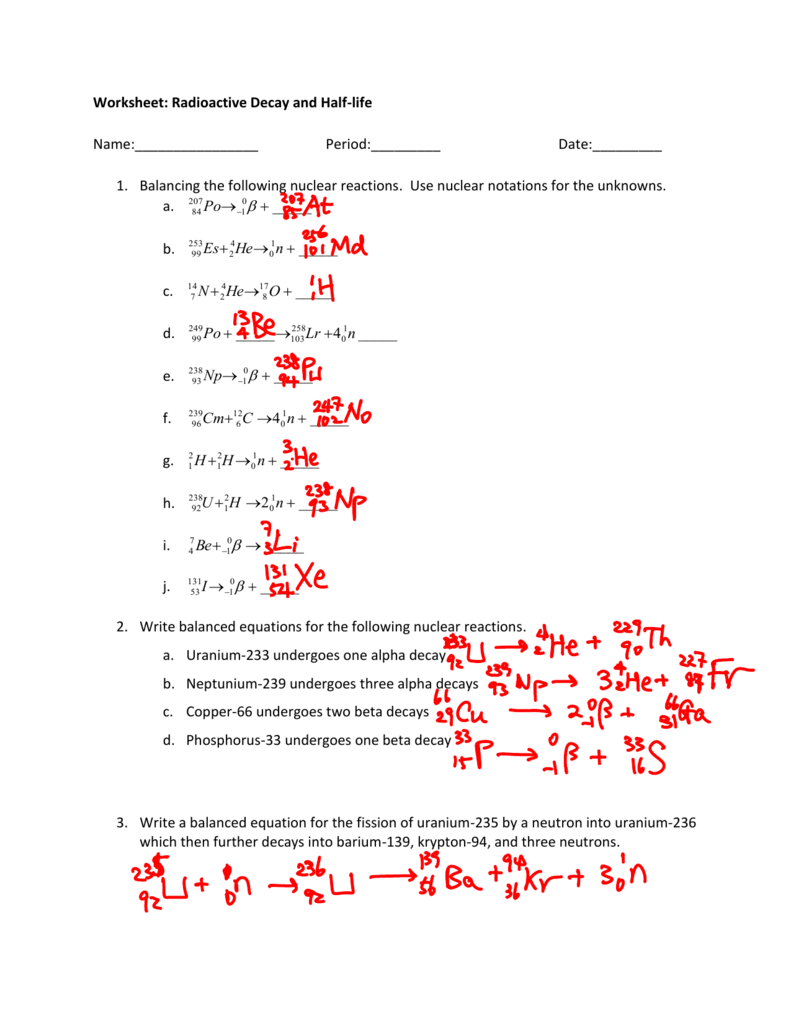
To balance a nuclear equation: - Ensure the sum of the atomic numbers (Z) on both sides of the equation is equal. - Ensure the sum of the mass numbers (A) on both sides of the equation is equal.
Practical Examples of Balancing Nuclear Reactions

Let’s explore some concrete examples:
Example 1: Beta Decay

Consider the beta decay of Carbon-14 (C-14) to Nitrogen-14 (N-14):
146C → 147N + 0-1e
Here, the atomic number increases from 6 to 7, while a beta particle (e) is emitted. Both mass and charge are conserved:
| Reaction | Atomic Number | Mass Number |
|---|---|---|
| C-14 | 6 | 14 |
| N-14 | 7 | 14 |
| β-particle (e) | -1 | 0 |
📌 Note: The atomic numbers sum to 6 (left side) + (-1) = 7 (right side). The mass numbers are balanced at 14 on both sides.
Example 2: Alpha Decay

Let’s examine the alpha decay of Uranium-238 (U-238) to Thorium-234 (Th-234):
23892U → 23490Th + 42He
Here, Uranium decays into Thorium with the emission of an alpha particle (Helium nucleus). The mass and charge conservation are as follows:
| Reaction | Atomic Number | Mass Number |
|---|---|---|
| U-238 | 92 | 238 |
| Th-234 | 90 | 234 |
| α-particle (He) | 2 | 4 |
📌 Note: The atomic numbers sum to 92 (left side) = 90 (Th) + 2 (He). The mass numbers are balanced at 238.
Advanced Techniques in Balancing Nuclear Equations

- Identify Unknowns: If an element or particle is unknown, determine its identity by balancing the equation.
- Track Neutron Emission: In some reactions, neutrons might be emitted or absorbed, affecting the balance.
- Consider Electron Capture: Occasionally, an electron from an atom’s inner shell is captured by the nucleus, which decreases the atomic number.
Summing up, mastering the balance of nuclear reactions necessitates a firm grasp of the conservation laws for mass and charge. With an array of nuclear phenomena from fission to decay, each reaction type poses unique balancing challenges. The examples provided highlight practical approaches to ensure these equations are correctly balanced, fostering an understanding crucial for students and professionals in nuclear chemistry. Mastering nuclear reactions expands our ability to comprehend natural processes, harness nuclear energy, and contribute to medical treatments like cancer therapy through radiation. It opens up avenues to understand the stars' energy production, examine the Earth's geological transformations, and secure our world's energy future.
What makes balancing nuclear reactions different from chemical reactions?

+
Nuclear reactions involve changes in the nucleus, like altering protons and neutrons, whereas chemical reactions involve only electron rearrangements. Thus, nuclear balancing focuses on mass numbers and atomic numbers, ensuring conservation of both.
Why do we need to balance nuclear reactions?

+
Balancing nuclear reactions ensures conservation of mass and charge, providing insight into the reaction’s mechanism, energy output, and potential applications in various scientific and industrial contexts.
Can you balance nuclear reactions that involve the emission of particles other than alpha, beta, or gamma?

+
Yes, reactions involving positron emission, neutron capture, or proton emission are also balanced in a similar manner by ensuring the conservation of mass and atomic numbers, though they involve different particles with unique properties.



