5 Simple Steps to Master Balancing Equations: GCSE Worksheet With Answers

Balancing chemical equations is a fundamental skill every GCSE Chemistry student must master. Understanding how to balance an equation not only helps in solving chemical reactions but also provides insights into the law of conservation of mass. Here, we'll explore five simple steps to balance equations, enriched with real-life examples, and provide you with a worksheet with answers to test your understanding.
Step 1: Identify Reactants and Products


Start by listing out all the reactants and products in the chemical equation. For instance:
- Reactants: H2, O2
- Products: H2O
Step 2: Count the Atoms

Count the number of atoms on each side of the equation:
- Hydrogen (H): Left side = 2, Right side = 2
- Oxygen (O): Left side = 2, Right side = 1
🔍 Note: Don’t forget to count all atoms including those in polyatomic ions as single entities.
Step 3: Use Coefficients to Balance

| Element | Reactants | Products | Coefficient |
|---|---|---|---|
| H | 2 | 2 | 1 (already balanced) |
| O | 2 | 1 | Place 2 before H2O to balance |

Step 4: Check Your Work

After placing coefficients, recount the atoms:
- Hydrogen (H): Left = 2, Right = 4
- Oxygen (O): Left = 2, Right = 2
Now, adjust the coefficient for H2 to 2 to balance hydrogen:
- 2H2 + O2 → 2H2O
🔍 Note: Verify that all elements are balanced, ensuring the law of conservation of mass holds.
Step 5: Simplify If Possible

If necessary, simplify the equation by dividing all coefficients by a common factor if it exists. In our case:
- 2H2 + O2 → 2H2O
Since there’s no common factor, the equation is already in its simplest form.
Now, let’s apply these steps to a worksheet to solidify your understanding.
Worksheet: Balancing Chemical Equations
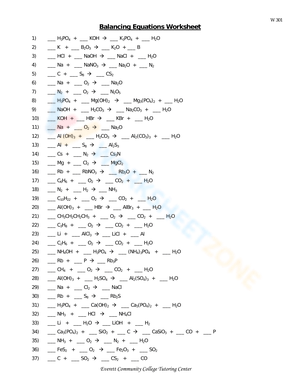
- Na + Cl2 → NaCl
- Fe + O2 → Fe2O3
- N2 + H2 → NH3
- K + H2O → KOH + H2
- Mg + O2 → MgO
Answers to Worksheet:

- 2Na + Cl2 → 2NaCl
- 4Fe + 3O2 → 2Fe2O3
- N2 + 3H2 → 2NH3
- 2K + 2H2O → 2KOH + H2
- 2Mg + O2 → 2MgO
These five steps provide a structured method to balance chemical equations, ensuring that you account for all atoms, balance them appropriately, and verify your work. Balancing equations will become second nature with practice, allowing you to confidently tackle more complex chemical reactions. Remember, patience and meticulous attention to detail are your allies in mastering this skill.
Why is it important to balance chemical equations?

+
Balancing chemical equations is essential because it reflects the law of conservation of mass, ensuring that matter is neither created nor destroyed in chemical reactions. This process helps chemists understand how reactants form products in exact quantities.
Can I balance equations in any order?
+
Yes, you can start balancing from any element as long as you ensure that all atoms are balanced in the end. However, there are conventional strategies like balancing the most complex molecule first or elements that appear only once on each side of the equation.
What if my equation cannot be balanced?

+
This is unlikely to happen with correct reactants and products. If you find an equation that seems unbalanceable, double-check your reactants and products or look for a potential reaction error. Sometimes, reactions need an additional condition or phase to balance.
How can I simplify balancing large equations?

+
For larger equations, start by balancing the least complex molecules or ions first. Use fractions if needed to balance elements that only appear once. Then multiply through to clear the fractions. Use algebraic methods or trial and error for complex balancing.



