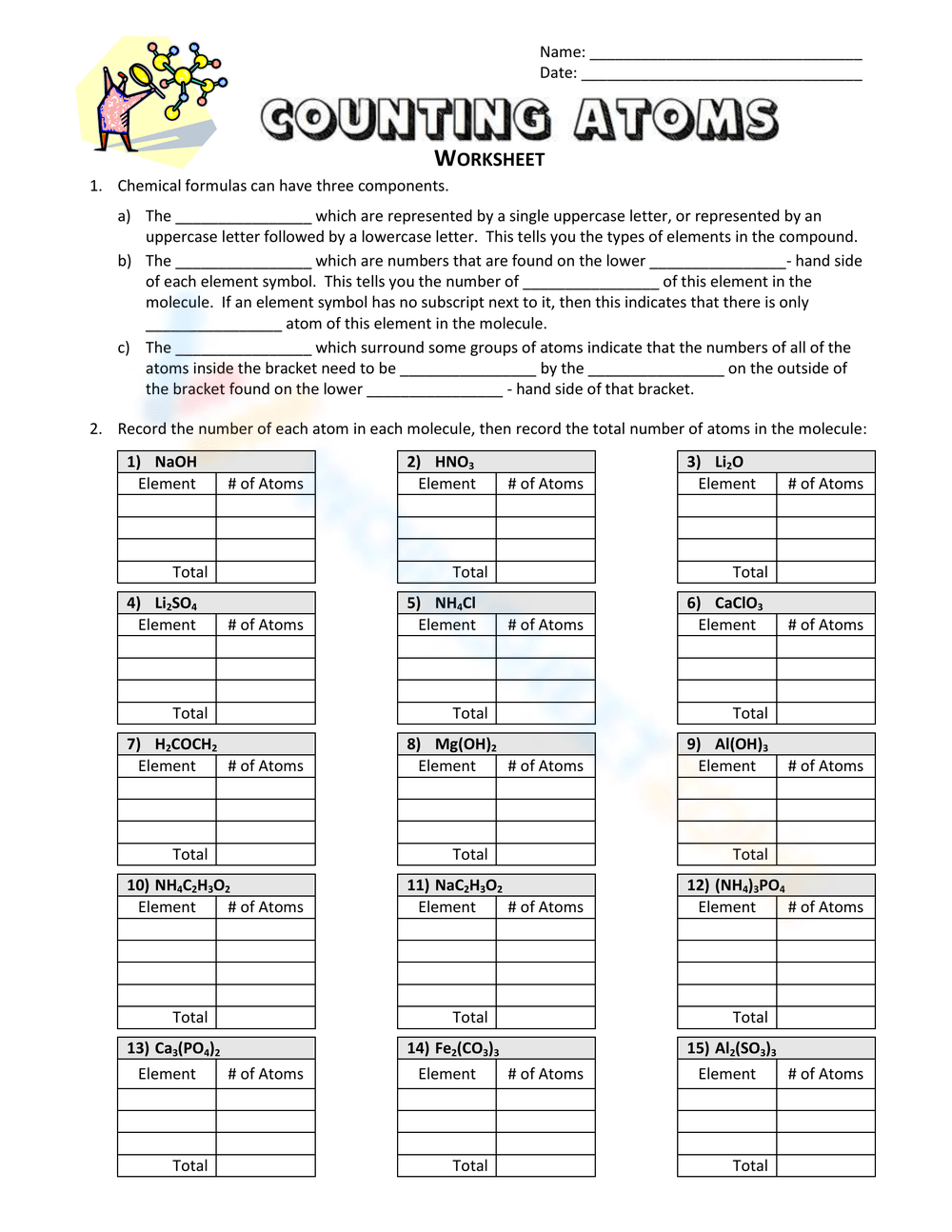
5 Proven Ways to Ace Your Counting Atoms Worksheet

Balancing chemical equations and understanding molecular composition is a fundamental skill in chemistry, making worksheets on counting atoms an essential practice for students at various educational levels. While these worksheets can initially seem daunting, there are proven strategies to help you excel. Here's how you can tackle those pesky counting atoms worksheets with confidence and precision.
1. Master the Basics of Chemical Formulas

Understanding the Language of Chemistry: Before you even begin counting atoms, it's crucial to comprehend the basics of how chemical formulas are written.
- Each element is represented by its chemical symbol from the periodic table.
- Subscripts show how many atoms of each element are in a molecule or compound.
- If there is no subscript after an element symbol, it means there's only one atom of that element.
- Parentheses can be used to group atoms together with a subscript applied to the entire group.
⚠️ Note: A common mistake is to overlook the numbers outside parentheses, which multiply the entire group inside the parentheses.
2. Use the Molar Mass Method

When tackling counting atoms worksheets, using the concept of molar mass can be immensely helpful:
- Calculate the molar mass of the compound by summing the atomic masses of its constituent elements.
- Determine how many moles of each element are in the molecule by dividing the molar mass of each element by the molecular mass of the compound.
- Multiply the moles of each element by Avogadro's number to find the number of atoms.
3. Create a Cheat Sheet

Here's how you can make an effective cheat sheet:
- List common elements with their symbols and atomic masses.
- Include examples of polyatomic ions with their charges.
- Summarize the rules for naming and writing chemical formulas.
- Use different colors for subscripts, coefficients, and element symbols for quick visual recognition.
📝 Note: Regularly updating your cheat sheet as you encounter new information can improve its effectiveness.
4. Practice Dimensional Analysis
Dimensional analysis, also known as the factor-label method, helps you keep track of units during calculations. Here are some steps to follow:
- Identify the known quantity and the units you need to convert to.
- Write conversion factors based on the information provided or known constants (e.g., Avogadro's number).
- Set up the equation by canceling out units and solve for the target unit.
5. Use Visual Aids

Visual aids can make abstract concepts more tangible:
- Draw or use 3D models of molecules to see the arrangement of atoms.
- Utilize color-coded diagrams or charts to quickly identify elements and their counts.
- Try apps or interactive online tools that allow you to build and count atoms in virtual molecules.
🖍️ Note: Visual aids are not just for beginners; even seasoned chemists use molecular modeling software to visualize complex structures.
By applying these methods, you'll not only improve your accuracy but also gain a deeper understanding of chemistry. Remember, the key to mastering counting atoms worksheets lies in practice, understanding, and the use of strategic learning tools. As you continue to practice these techniques, you'll find your comfort and proficiency with chemical formulas and their constituents increase significantly.
Why is understanding chemical formulas important?

+
Understanding chemical formulas is crucial because it’s the basis for all chemical reactions and processes. It helps in determining the composition, properties, and reactions of compounds.
How can I remember the number of atoms in each compound?

+
Creating flashcards with common compounds on one side and the count of atoms on the other can help. Additionally, regularly practicing by writing formulas and atom counts can reinforce this skill.
What if I forget how to balance a chemical equation?

+
Balancing equations is a skill that improves with practice. There are many online tools and apps that can help you balance equations and provide step-by-step explanations for learning and practice.
Is there a quick way to check my work on counting atoms?

+
You can cross-check by working backwards from the total molecular mass to ensure the sum of atomic masses corresponds with the number of atoms you’ve counted.