Avogadro's Law Worksheet Answers: Ace Chemistry Simplified

Avogadro's Law, named after the Italian scientist Amedeo Avogadro, provides a fundamental insight into the behavior of gases at the molecular level. This principle states that equal volumes of all gases, at the same temperature and pressure, contain an equal number of molecules. This understanding opens up a range of practical applications in chemistry, from determining molecular weights to analyzing gas reactions.
Understanding Avogadro’s Law

Before diving into the worksheets and answers, let’s establish a solid understanding of Avogadro’s Law:
- Constant Temperature and Pressure: The law holds true only when temperature and pressure remain constant.
- Volume and Number of Moles: There is a direct proportionality between the volume of a gas and the number of moles of gas, given the conditions above. This relationship can be expressed as:
V1/n1 = V2/n2
Where V represents volume and n represents the number of moles of gas.
Worksheet Walkthrough with Examples
Let’s tackle some common problems from Avogadro’s Law worksheets:
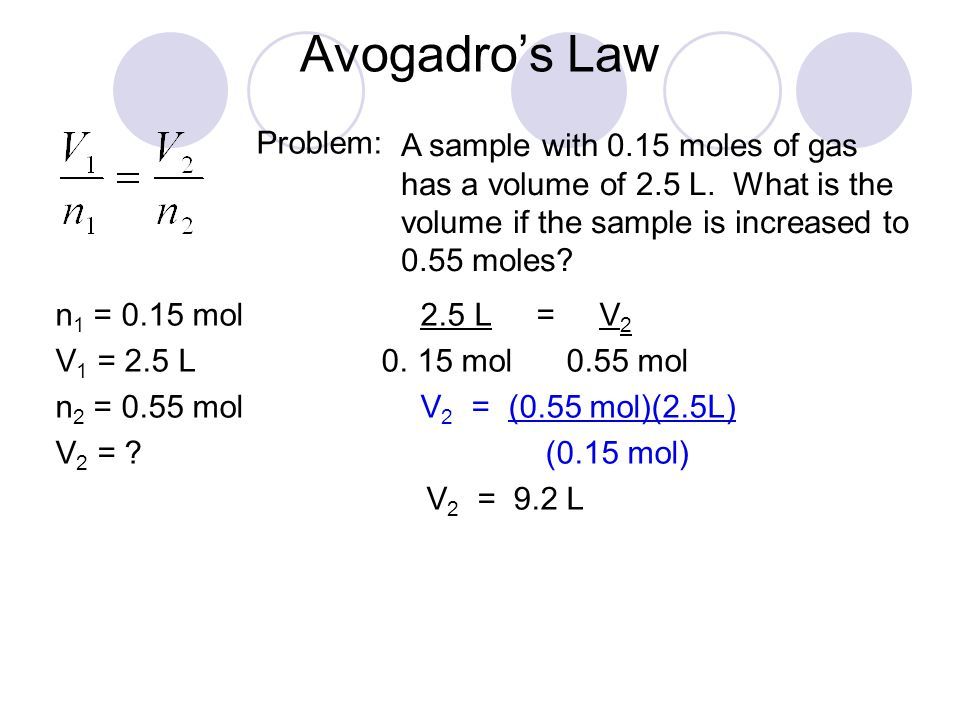
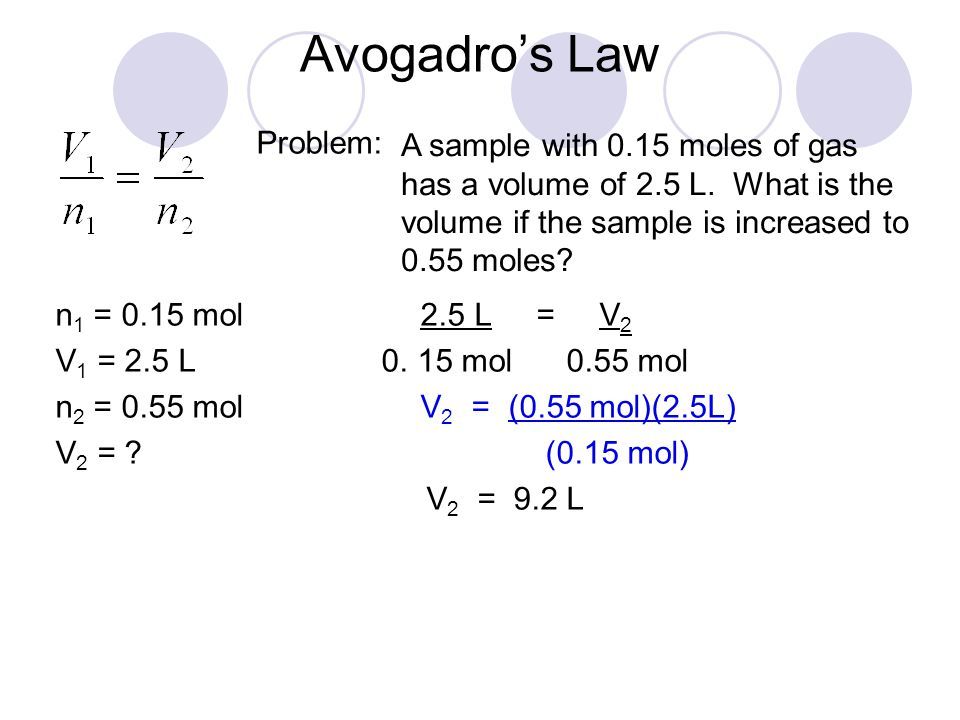
Example 1: Simple Ratio Calculation

If 2.0 moles of a gas occupy 44.8 liters at STP (Standard Temperature and Pressure), how many liters will 1.0 moles of the same gas occupy under the same conditions?
- Using Avogadro’s Law:
V1/n1 = V2/n2
V2 = (V1 * n2) / n1 = (44.8 L * 1.0 mol) / 2.0 mol = 22.4 L
💡 Note: Remember, STP conditions are 0°C (273.15 K) and 1 atm (101.3 kPa).
Example 2: Comparison of Gases
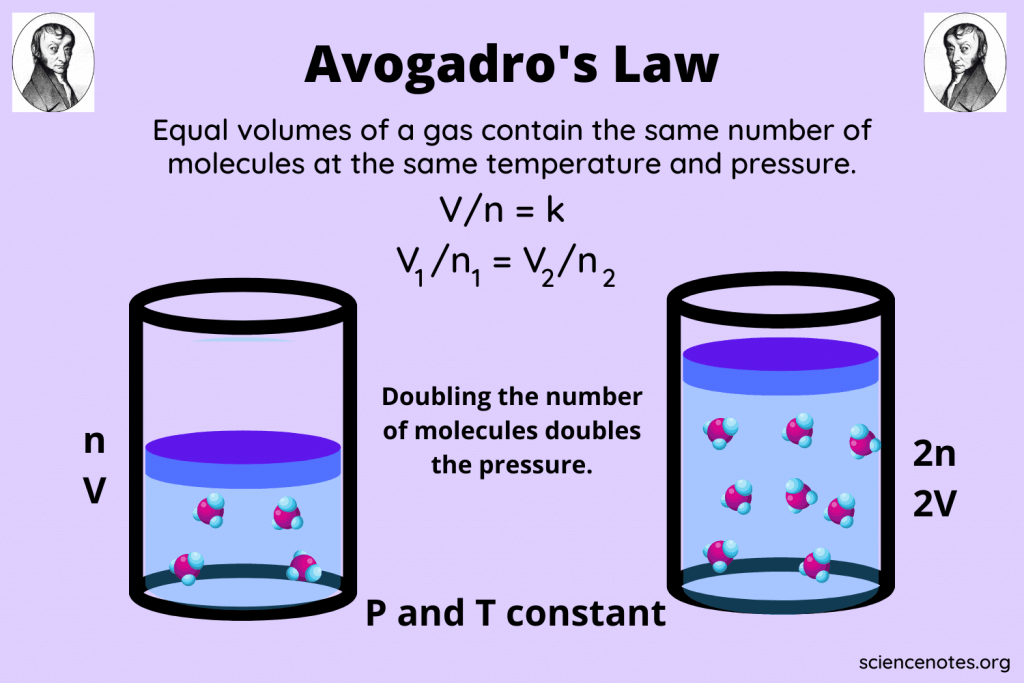
Given that 5.0 liters of O2 at STP is equal to how many moles of N2?
- Using Avogadro’s Law:
n1/V1 = n2/V2
n2 = (n1 * V2) / V1 = (n1 * 5.0 L) / 5.0 L = n1
Here, n1 and n2 are equal because the volumes are the same at STP, illustrating that the number of moles is identical for equal volumes of different gases under these conditions.
Practical Applications in Laboratory and Industry

Avogadro’s Law has several real-world applications:
- Gas Collection: In quantitative analysis, gases are collected over water or through displacement. Knowing the volume of gas allows for direct determination of the number of moles.
- Chemical Reactions: It helps predict the volume of gases produced or consumed in reactions, useful for scaling processes in chemical manufacturing.
🧪 Note: When collecting gases over water, remember to account for water vapor pressure using Dalton’s Law of Partial Pressures.
Summary and Key Takeaways
Avogadro’s Law is not just about numbers and ratios; it’s a gateway to understanding how gases behave at a microscopic level. The law’s elegance lies in its simplicity, providing a clear relationship between volume and moles of gas under constant conditions. Here are the key points to remember:
- Equal volumes of gases contain equal numbers of molecules at the same temperature and pressure.
- The volume of a gas is directly proportional to the number of moles of gas present.
- This principle is used in stoichiometric calculations involving gases, predicting product volumes, and understanding gas behavior in various conditions.
The concept of Avogadro’s Law simplifies complex gas interactions, enabling chemists to perform calculations with ease and accuracy.
What does Avogadro’s Law tell us about gas volume and number of particles?

+
Avogadro’s Law states that equal volumes of all gases, at the same temperature and pressure, have an equal number of molecules. This means the volume of a gas (V) is directly proportional to the number of moles of gas (n) under constant conditions.
Can Avogadro’s Law be applied if temperature or pressure changes?
+
No, Avogadro’s Law specifically applies when temperature and pressure remain constant. If either changes, you would need to use the Combined Gas Law or Ideal Gas Law to account for these variations.
How does Avogadro’s Law help in determining the molecular weight of gases?
+
Since we know the volume and moles of a gas at STP, we can use Avogadro’s Law along with the molar volume of any gas (22.4 L/mol at STP) to calculate molecular weights of gases by comparing their volumes to their known number of moles.