Oxidation Numbers Worksheet: Ultimate Answer Key Guide
Understanding oxidation numbers is a fundamental concept in chemistry that can help you predict the behavior of elements in a wide variety of chemical reactions. This guide will delve deep into the realm of oxidation numbers, offering detailed explanations, step-by-step examples, and common pitfalls to avoid. Whether you're a student tackling redox reactions for the first time or a seasoned chemist looking to brush up on your basics, this ultimate answer key guide will ensure you have all the tools at your disposal.
Why Oxidation Numbers Matter

Oxidation numbers (also known as oxidation states) are essentially an accounting tool for electrons during a chemical reaction. Here's why they're important:
- Reaction Prediction: Oxidation numbers can help predict whether a substance will gain or lose electrons, which is crucial for understanding reactions like combustion, corrosion, and photosynthesis.
- Balancing Redox Equations: They are essential for balancing chemical equations where the oxidation states of atoms change, as in redox (reduction-oxidation) reactions.
- Electrochemistry: Oxidation numbers are key in electrochemistry, where they dictate the flow of electrons in batteries, fuel cells, and electrolytic cells.
- Chemical Bonding: Understanding oxidation states can provide insights into the type of bonds atoms form, whether they are ionic or covalent.
How to Assign Oxidation Numbers
Here are the rules for assigning oxidation numbers:
Basic Rules:

- Uncombined Elements: In their elemental form (e.g., H2, O2, N2), oxidation numbers are always 0.
- Monatomic Ions: The oxidation number of a monatomic ion is equal to its ionic charge (e.g., Na+ has an oxidation number of +1).
- Oxygen: Usually, oxygen has an oxidation state of -2 in compounds, except in peroxides where it is -1, and in fluorides like OF2 where it is +2.
- Hydrogen: When bonded with non-metals, hydrogen has an oxidation number of +1, and with metals (hydride ions), it’s -1.
- Fluorine: Fluorine always has an oxidation number of -1 in all its compounds.
Compounds and Polyatomic Ions:

- The sum of the oxidation numbers in a neutral compound must be zero.
- In a polyatomic ion, the sum of oxidation numbers equals the ion’s charge.
- Group IA and IIA metals: Alkali and alkaline earth metals often have oxidation states of +1 and +2, respectively, unless they are in unusual states like in some complex compounds.
⚠️ Note: Some elements can have several common oxidation states. For instance, iron can be in +2 or +3 states; transition metals are notorious for this!
Examples:

- NO:
- Assign oxygen its typical -2 oxidation state.
- Let the oxidation number of N be x. Thus, we have:
- x + (-2) = 0 => x = +2
- H2O:
- Hydrogen is +1, and oxygen -2.
- The equation: 2(+1) + (-2) = 0
- MnO4-:
- The sum of oxidation numbers equals the ion’s charge (-1).
- 4 Oxygen atoms are -2 each, so -8 in total.
- Let Mn be x, then: x + (-8) = -1 => x = +7
✅ Note: When dealing with polyatomic ions like MnO4-, ensure to account for the ion's charge.
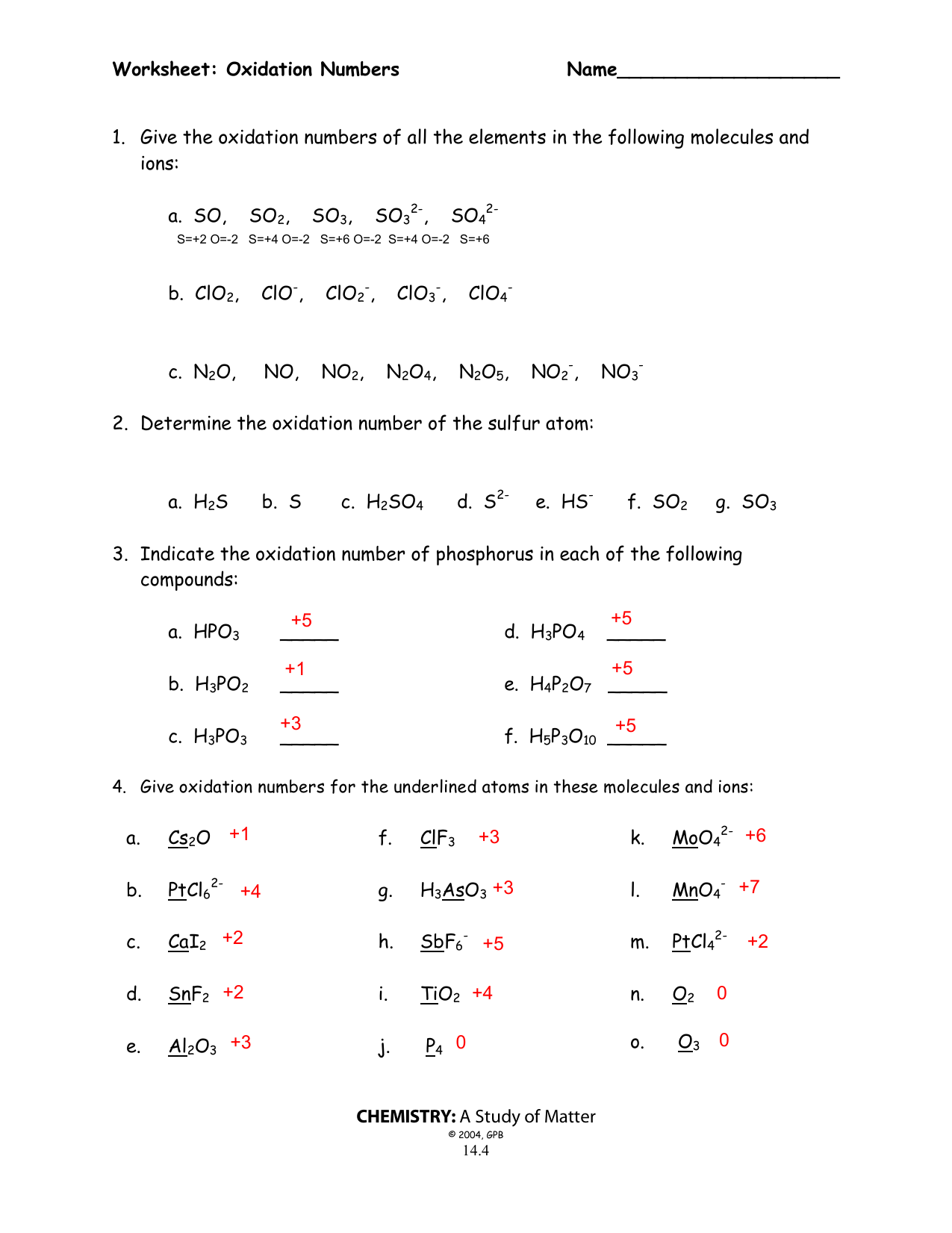
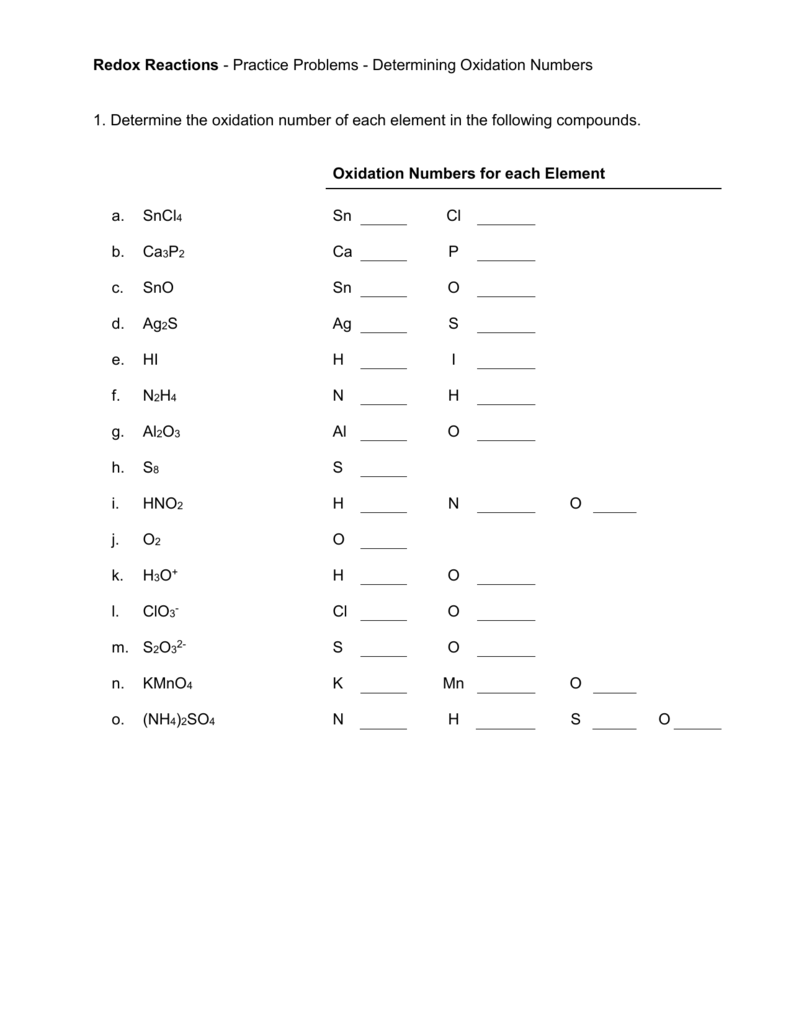
Worksheet and Ultimate Answer Key

| Compound | Element | Oxidation Number |
|---|---|---|
| H2O | H | +1 |
| H2O | O | -2 |
| CO2 | C | +4 |
| CO2 | O | -2 |
| Fe2O3 | Fe | +3 |
| Fe2O3 | O | -2 |
| CaCl2 | Ca | +2 |
| CaCl2 | Cl | -1 |
| NaNO3 | Na | +1 |
| NaNO3 | N | +5 |
| NaNO3 | O | -2 |

📚 Note: Practice is key. Regularly working through worksheets helps in internalizing the rules and exceptions of oxidation numbers.
Common Mistakes and Tips

Here are some common mistakes to avoid and tips to remember:
- Assuming all metals have a single oxidation state: Be aware that transition metals can have multiple oxidation states.
- Misunderstanding zero oxidation state in diatomic molecules: Remember, elements in their molecular form always have an oxidation number of 0.
- Incorrectly balancing ionic compounds: In ionic compounds, the sum of oxidation numbers must equal the net charge.
- Tip: When dealing with complex compounds, try to identify the oxidation number of elements with a known or more straightforward state first, then calculate the others.
- Tip: Consider the charge on any ions or radicals when assigning oxidation numbers.
💡 Note: Always double-check your work. In chemistry, one wrong oxidation state can mislead the entire reaction analysis!
What is the difference between oxidation state and oxidation number?

+
While often used interchangeably, oxidation state refers to the degree of oxidation (loss of electrons) of an atom in a chemical compound, while oxidation number is a more formal book-keeping charge assigned according to a set of rules to reflect the electron distribution.
Why do some elements have multiple oxidation states?

+
Elements, especially transition metals, can have multiple oxidation states due to the availability of multiple valence shells or the ability to form complex ions where electrons can move between energy levels.
How can knowing oxidation numbers help in balancing chemical equations?

+
In redox reactions, oxidation numbers provide a clear way to track electron transfer. By ensuring the total increase in oxidation number equals the total decrease, one can balance the equation accurately.
Can oxidation states be fractional?

+
In most practical chemistry, oxidation states are whole numbers because electrons can only be lost or gained as whole units. However, in certain theoretical or simplified models, especially in mixed valence compounds, fractional oxidation states can be assigned for convenience.
What does a change in oxidation number indicate in a reaction?

+
A change in oxidation number indicates that a redox reaction has occurred. An increase in oxidation number means an atom has been oxidized (lost electrons), while a decrease means it has been reduced (gained electrons).