Mastering Atom Counting: Free Worksheet and Tips

Counting atoms is a fundamental skill in chemistry that forms the bedrock for understanding chemical equations, stoichiometry, and the mass relationships in chemical reactions. This skill allows students and professionals alike to dissect the molecular structure of compounds, predict reaction outcomes, and work out molar ratios. Here, we'll dive deep into the nuances of atom counting with a free worksheet, useful tips, and insights to help you master this essential chemical literacy.
Understanding Atomic Composition

Atoms are the smallest unit of an element that retains the chemical properties of that element. When different atoms combine in specific proportions, they form compounds. To understand atom counting:
- Learn the basics of atomic symbols and molecular formulas.
- Get acquainted with subscript and coefficient notation.
- Recognize polyatomic ions and their charges.
Why Master Atom Counting?
Here's why mastering atom counting is not just about numbers:
- Quantitative Analysis: It allows for the quantitative analysis of substances in chemical reactions.
- Reaction Prediction: Understanding how atoms combine helps in predicting how compounds will react.
- Chemical Equation Balancing: Atom counting is crucial for balancing equations, ensuring the law of conservation of mass is upheld.
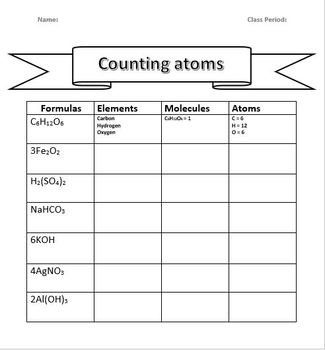
Your Free Atom Counting Worksheet
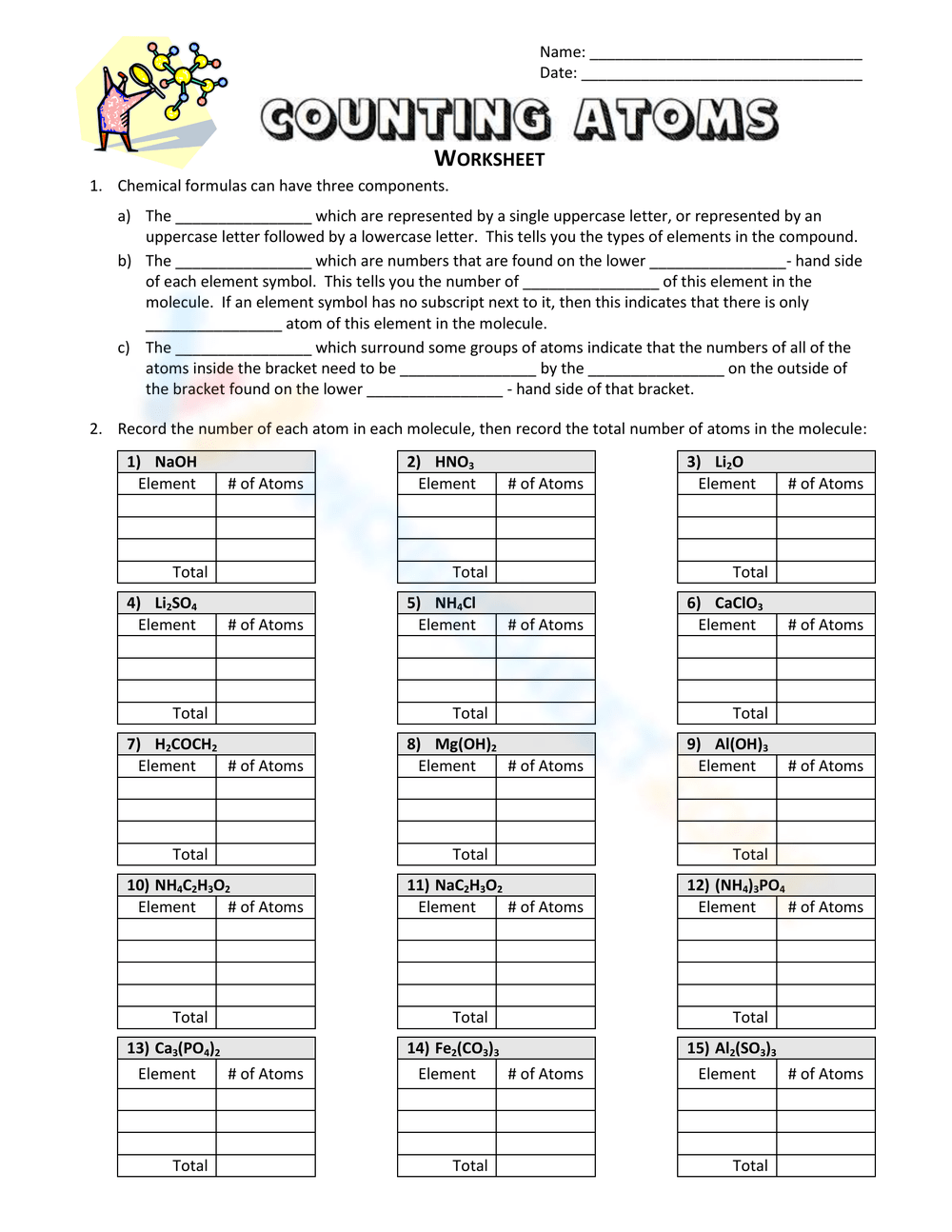
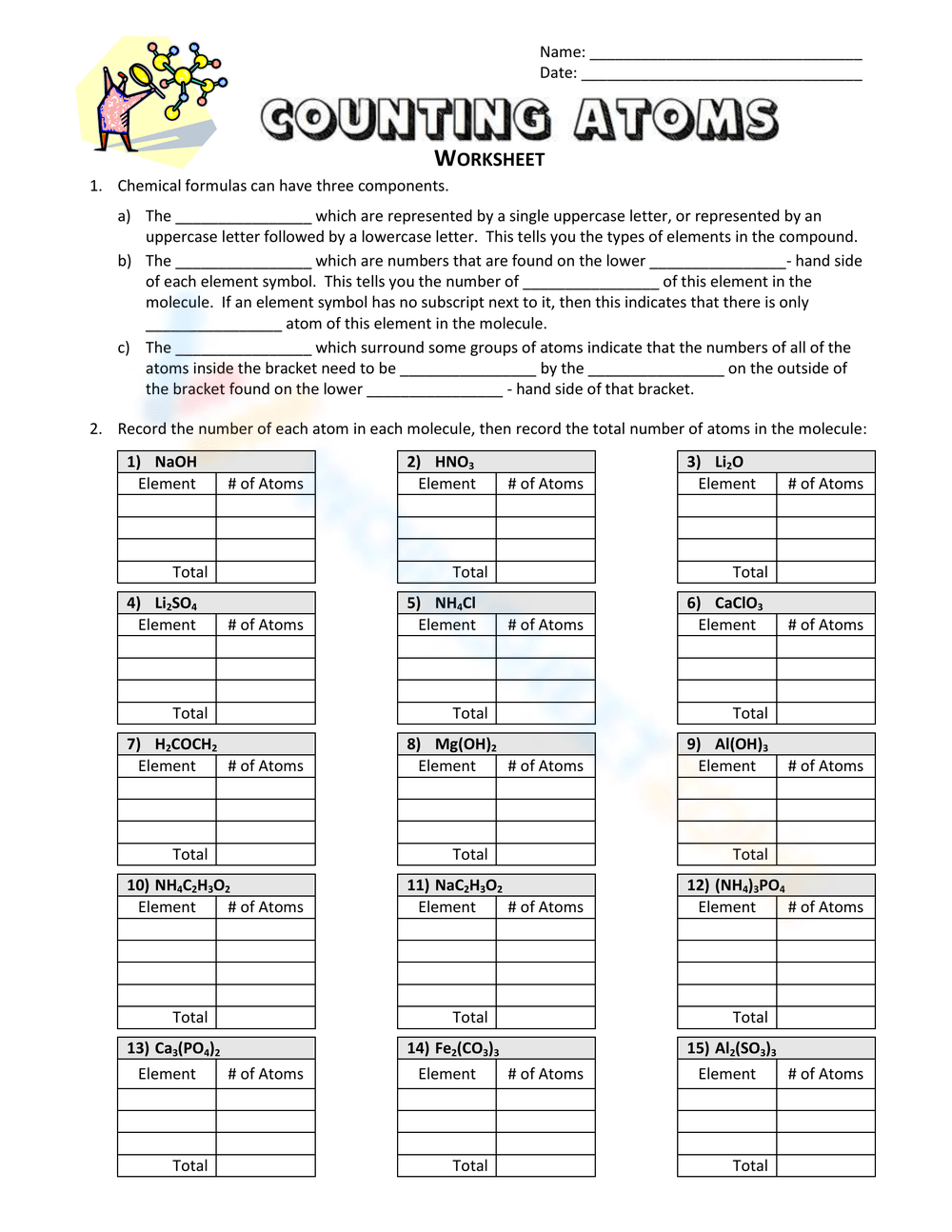
To help you practice, here is a worksheet designed to enhance your atom counting skills:
| Compound | Number of Each Atom |
|---|---|
| H2O | H: 2, O: 1 |
| NaOH | Na: 1, O: 1, H: 1 |
| (NH4)2SO4 | N: 2, H: 8, S: 1, O: 4 |
| C6H12O6 | C: 6, H: 12, O: 6 |

📘 Note: This is just a sample. More extensive worksheets can be created by following the tips below.
Tips for Mastering Atom Counting
Identify the Formula Units

- Break down the compound into smaller parts. If there is a parenthesis with a number outside, multiply all atoms within by that number.
Count Single Atoms First

- Start by identifying individual atoms before tackling polyatomic ions.
Use Moles and Avogadro’s Number
- Link your atom count with moles. Remember, one mole of any substance contains Avogadro’s number (6.022 × 10^23) of atoms.
Memorize Common Polyatomic Ions

- Familiarize yourself with ions like sulfate (SO4^2-), nitrate (NO3^-), and others to quickly identify atom counts within these groups.
Practice, Practice, Practice

- Repetition is key. Make your own worksheet or download ones available online.
🧪 Note: When working with complex molecules, breaking them down into parts can simplify the counting process.
Atom Counting in Practice

Let’s work through an example:
(NH4)3PO4
- N: 3 * 1 = 3
- H: 3 * 4 = 12
- P: 1 * 1 = 1
- O: 4 * 1 = 4
Conclusion

Atom counting is more than a mere academic exercise; it is a tool that opens up the world of chemistry, allowing us to dissect and understand the very nature of matter. By practicing with worksheets and following these tips, you’re not just learning to count atoms; you’re developing a deeper comprehension of chemical interactions and processes. This skill will be invaluable as you progress through your studies or work in any chemistry-related field. Keep honing your skills, and soon, atom counting will become second nature, enhancing your ability to tackle even the most complex chemical problems.
What are polyatomic ions, and why are they important for atom counting?

+
Polyatomic ions are charged species composed of two or more atoms covalently bonded together. They are important because they act as single units in compounds, affecting how we count atoms within these units. Recognizing these ions simplifies atom counting in complex compounds.
How does one balance a chemical equation using atom counting?
+
To balance an equation, you must ensure that the number of atoms for each element is the same on both sides of the reaction arrow. Atom counting helps you adjust the coefficients (the numbers before the compound formulas) to achieve this balance.
Can atom counting help in understanding reaction mechanisms?

+
Yes, knowing how many and which atoms are involved in a reaction can provide clues about the steps through which reactants transform into products. It’s a crucial part of understanding how reactions proceed at the molecular level.