Solubility Rules Worksheet: Comprehensive Answer Key

The concept of solubility is fundamental in chemistry, affecting everything from pharmaceutical manufacturing to environmental cleanup. Understanding how and why substances dissolve in solvents is not only fascinating but essential for anyone in the sciences. This post will delve deep into solubility rules, providing a detailed solubility rules worksheet answer key to clarify common questions and enhance your understanding of chemical solubility principles.
What is Solubility?

At its core, solubility describes the ability of a substance (the solute) to dissolve in a solvent to form a solution. Solubility can be measured in grams of solute per liter of solvent or moles per liter (molarity), giving us an idea of how much solute can dissolve at a given temperature.
The solubility of a substance depends on several factors:
- Temperature: Generally, solubility increases with temperature, especially for solids in liquids.
- Pressure: Solubility of gases in liquids increases with pressure (Henry's Law).
- Chemical Nature: Like dissolves like – ionic or polar substances dissolve well in polar solvents, while nonpolar substances dissolve in nonpolar solvents.
- Intermolecular Forces: The type and strength of forces between solute and solvent particles significantly influence solubility.
General Solubility Rules
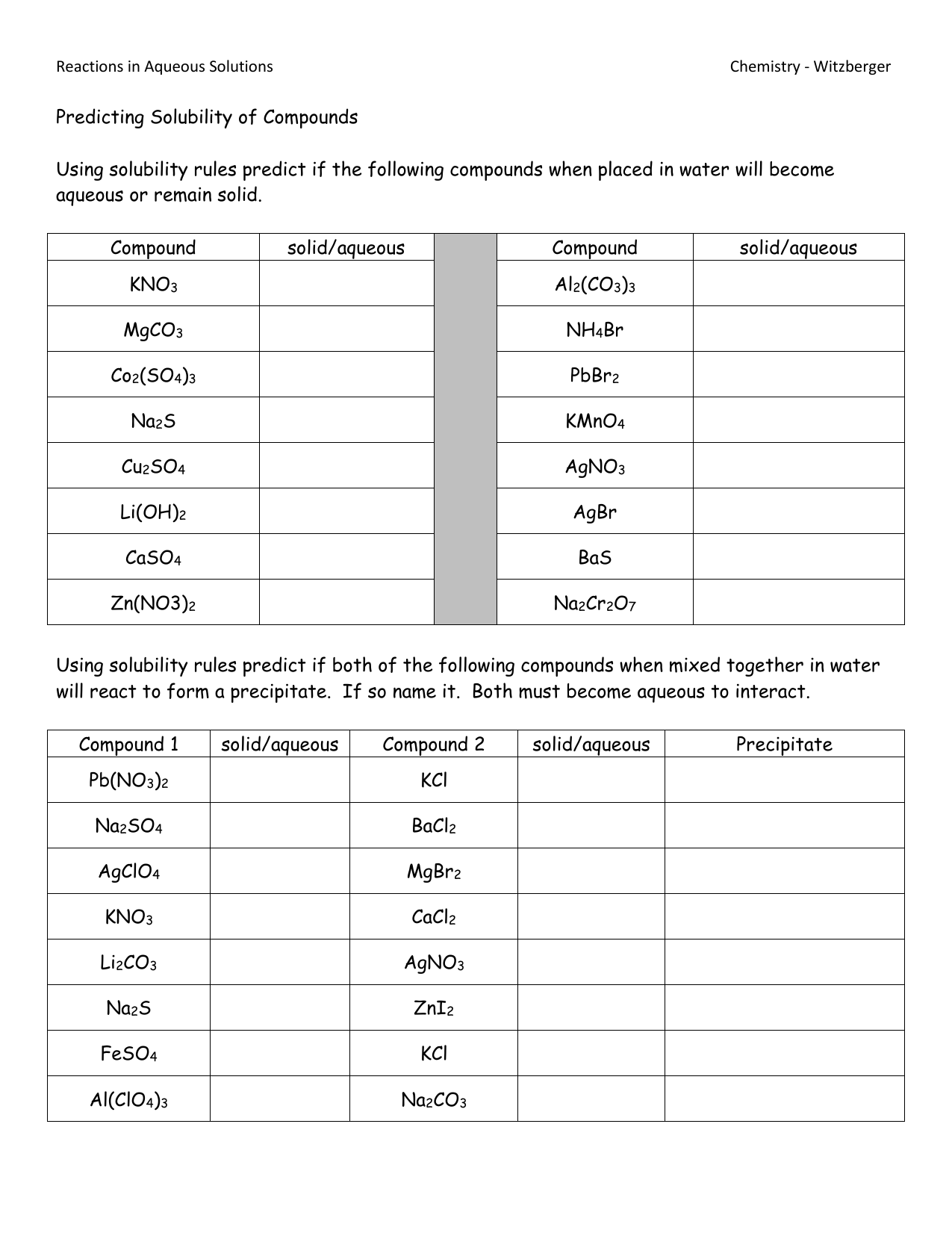
Here's a basic overview of common solubility rules that can serve as a guideline:
| Ion | Solubility in Water |
|---|---|
| NO3- | Soluble |
| Cl-, Br-, I- (except with Ag+, Hg22+, Pb2+) | Soluble |
| SO42- (except with Ca2+, Sr2+, Ba2+, Ag+, Pb2+) | Soluble |
| NH4+ | Soluble |
| Most others | Insoluble |
🔍 Note: Keep in mind that these are general rules. Exceptions exist, especially under specific conditions or with certain ions.
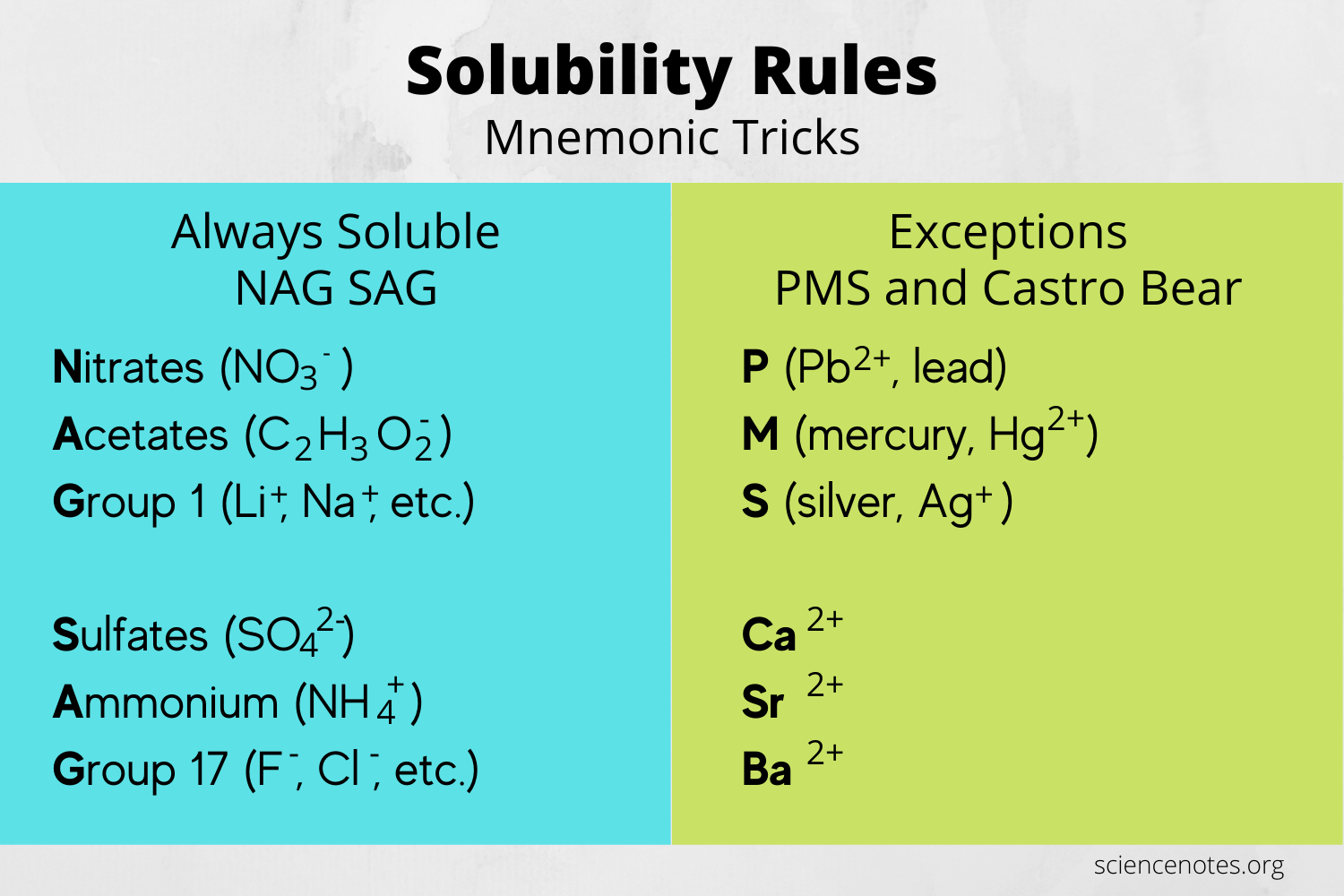
Solubility Rules Worksheet: Detailed Answers

In this section, we'll address common questions found in solubility rules worksheets, providing comprehensive explanations to help you master the topic:
- Question: Is PbSO4 soluble in water?
- Answer: No. According to the solubility rules, PbSO4 falls under the exception for sulfate compounds, making it insoluble.
- Question: Are all nitrates soluble?
- Answer: Yes, all nitrates are soluble in water. Nitrates have no common exceptions.
- Question: How does temperature affect the solubility of solids?
- Answer: For most solids, solubility increases with increasing temperature. This is due to the increase in kinetic energy, which enhances the interaction between solute and solvent particles.
- Question: Is AgCl soluble?
- Answer: No. Silver chloride (AgCl) is one of the exceptions where chlorides are typically soluble, thus it is insoluble in water.
- Question: How can you increase the solubility of a gas in water?
- Answer: Increase the pressure of the gas or lower the temperature. According to Henry's Law, the solubility of gases in liquids is directly proportional to the pressure of the gas.
💡 Note: Always check the specific temperature and pressure conditions if provided, as these can significantly alter solubility predictions.
Conclusion Paragraph
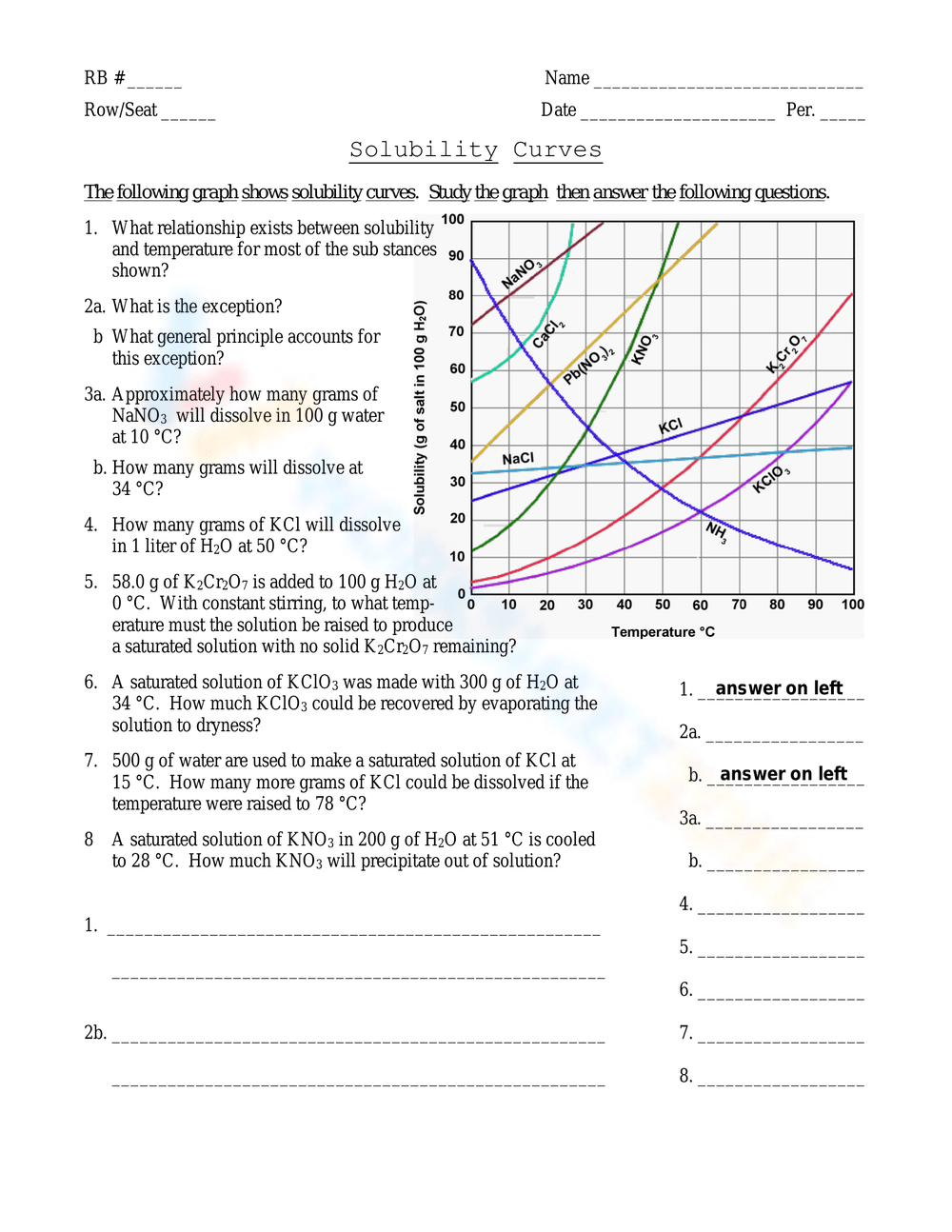
In summary, the world of solubility provides a fascinating glimpse into the interactions at the molecular level. We've explored the key principles that govern whether a substance will dissolve or not, highlighting essential solubility rules, exceptions, and even how to manipulate solubility through changes in temperature and pressure. Understanding these rules not only aids in academic studies but also has practical applications in numerous fields. We encourage you to apply these principles in real-world scenarios, where solubility plays a critical role in processes ranging from cooking to advanced chemical engineering.
What does it mean for a substance to be soluble?

+
When a substance is described as soluble, it means it can dissolve in a solvent, like water, to form a homogeneous solution.
Can you always predict solubility with rules?
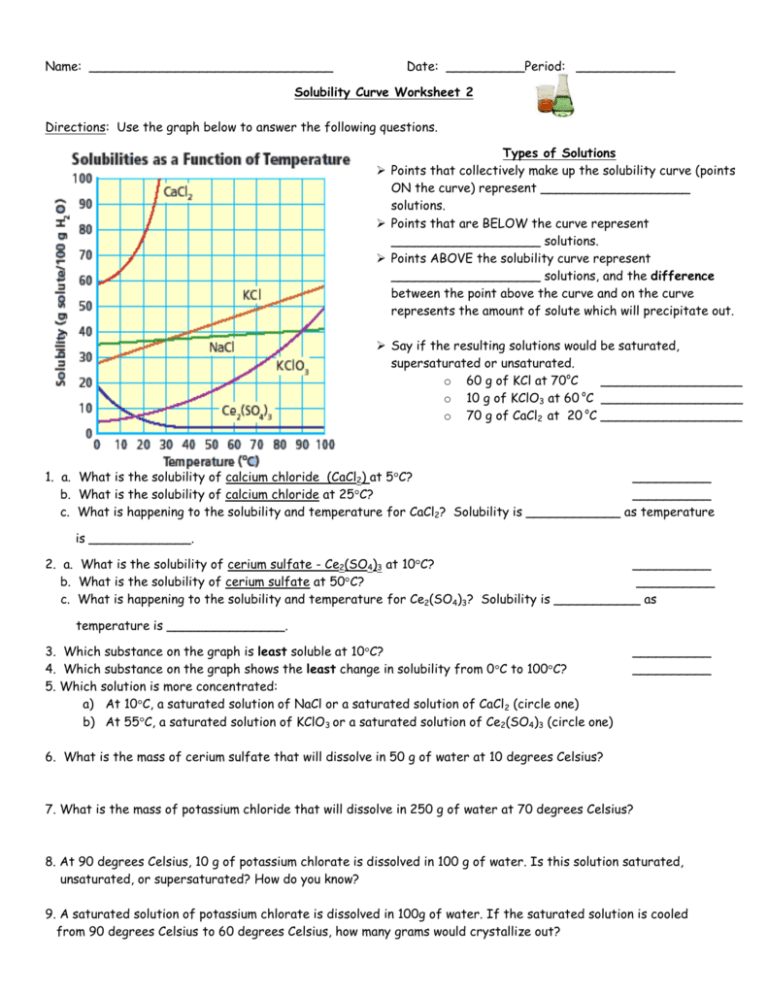
+
Solubility rules provide a general guideline. There are exceptions due to unique chemical properties or specific conditions like pH or presence of other ions.
What factors influence the solubility of a substance?

+
The main factors are the chemical nature of both the solute and solvent, temperature, pressure (especially for gases), and the strength of intermolecular forces.
Are there substances that don’t follow solubility rules?
+
Yes, some substances exhibit complex behavior due to unique chemical properties or the presence of certain ions, making their solubility unpredictable by simple rules.