5 Ways to Master Word and Skeleton Equations

In the realm of chemistry, understanding and mastering the art of balancing chemical equations is crucial for students and professionals alike. Word and skeleton equations form the foundation of this knowledge. This blog post will delve into the five essential ways to master these equations, providing you with the tools and techniques to excel in chemical equation balancing. Whether you're preparing for an exam or seeking to enhance your understanding of chemistry, these methods will ensure you're well-equipped.
The Basics of Word and Skeleton Equations
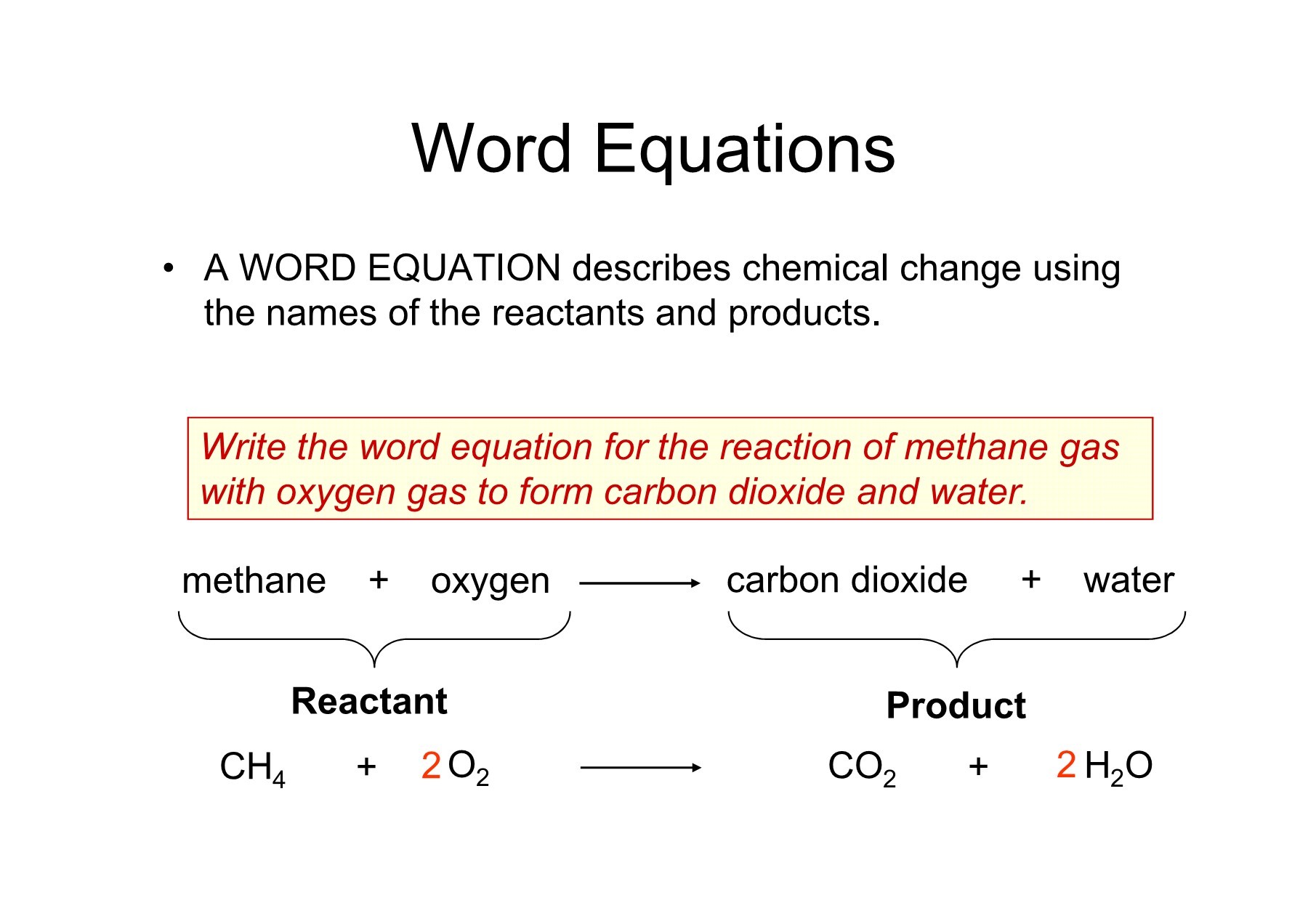
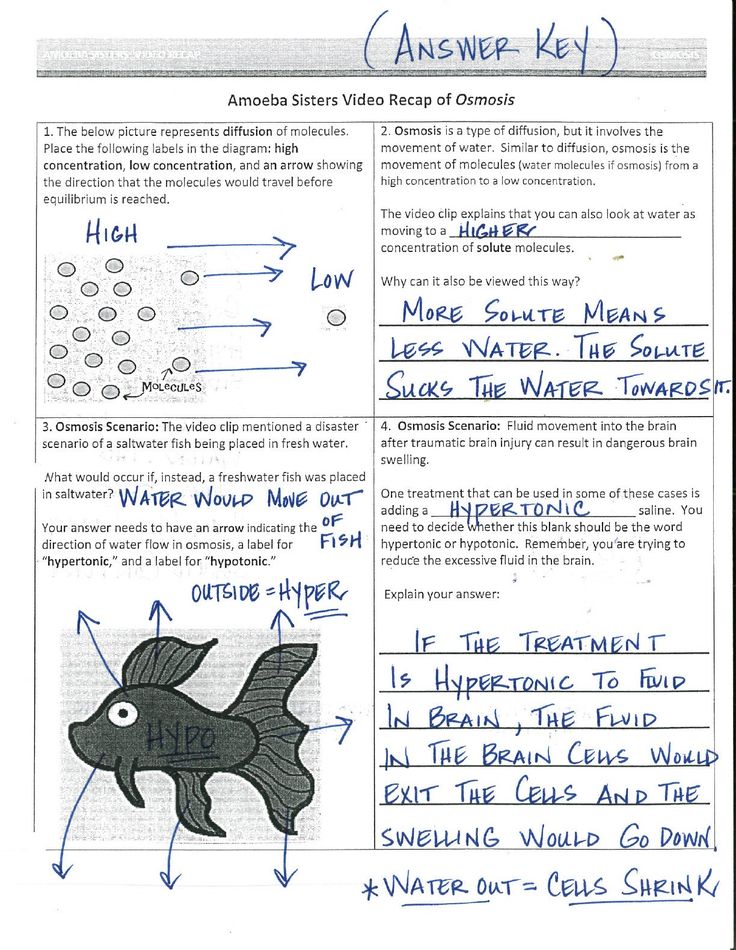
Before diving into mastering techniques, it's vital to grasp the basics. A word equation describes a chemical reaction in terms of the names of substances involved, without focusing on their chemical formulas or balancing. For example, the word equation for the reaction between iron and oxygen could be:
- Iron + Oxygen → Iron oxide
A skeleton equation, or unbalanced chemical equation, takes this a step further by using chemical formulas, but without ensuring the numbers of atoms on both sides are equal:
- Fe + O2 → Fe2O3
1. Understand the Law of Conservation of Mass

The first step in mastering word and skeleton equations is to understand the Law of Conservation of Mass. This law states that matter is neither created nor destroyed in a chemical reaction. Therefore, the total number of atoms of each element must be the same before and after the reaction. Here's how you apply this principle:
- Write Down the Skeleton Equation: Begin by listing the reactants and products in terms of their chemical formulas.
- Count Atoms: Examine the number of atoms on both sides of the equation. This initial check helps identify imbalances.
- Adjust Coefficients: Alter the coefficients in front of the formulas to balance the equation, ensuring no element is missing or in excess.
2. Use the Inspection Method for Simple Balancing

The inspection method, also known as the trial-and-error method, is the simplest approach for balancing basic chemical equations. Here's how it works:
- Start with the Most Complex Compound: Look for the compound with the greatest number of atoms or the most difficult to balance.
- Balance One Element at a Time: Work on one element, adjusting coefficients until you achieve balance.
- Check and Recheck: After each change, ensure no new imbalances have been introduced.
This method is straightforward but can become cumbersome for more complex reactions.
3. Implement the Algebraic Method for Complex Equations

For equations where simple inspection fails, the algebraic method comes into play. This method involves:
- Assign Variables to Coefficients: Place letters (e.g., a, b, c, d) as coefficients in front of each chemical species.
- Set Up Equations: For each element, set up an equation based on the atom count, ensuring coefficients balance on both sides.
- Solve the System: Solve these simultaneous equations to find the values of the coefficients.
- Ensure Rational Numbers: If solutions are fractional, multiply through to ensure whole numbers for coefficients.
🔍 Note: This method is particularly useful for polyatomic ions or when there's a large difference in atom count between reactants and products.
4. Leverage Visual Aids and Mnemonics
Visual learning tools and mnemonic devices can significantly improve your understanding and memory retention of chemical equations:
- Reaction Diagrams: Create simple diagrams to represent the reactants and products, showing the transformation visually.
- Mnemonics: Create phrases or words that help you remember reactions or their balancing.
- Color Coding: Use colors to group elements or compounds, making it easier to track atoms during balancing.
📝 Note: Visual aids can particularly help with spatial learners or those who struggle with abstract mathematical thinking.
5. Practice, Practice, Practice

Like any skill, proficiency in balancing chemical equations comes with practice:
- Engage in Drills: Use online resources, textbooks, or practice sheets designed for chemical equation balancing.
- Set Challenges: Try to balance equations under time constraints to simulate exam conditions.
- Analyze Common Reactions: Familiarize yourself with common reactions and their balancing to speed up your process.
Each equation you balance will reinforce your understanding of chemical reactions, helping you internalize the principles and techniques required for mastery.
🧪 Note: Regular practice not only enhances speed but also helps in recognizing patterns and exceptions in chemical reactions.
Mastering word and skeleton equations involves a combination of understanding basic principles like the Law of Conservation of Mass, using practical methods for balancing, employing visual aids for learning, and committing to consistent practice. By integrating these five ways into your study routine, you'll be able to confidently tackle any chemical equation you come across, whether in a lab, classroom, or on an exam. The journey to mastering these equations is one of both intellectual curiosity and practical application, enabling you to decode the language of chemistry with ease and proficiency.
What is the difference between a word equation and a skeleton equation?

+
A word equation describes a chemical reaction using the names of the substances involved without their chemical formulas. A skeleton equation, on the other hand, uses chemical formulas but isn’t necessarily balanced.
Why is balancing equations important in chemistry?

+
Balancing equations is essential to adhere to the Law of Conservation of Mass, which ensures that the number of atoms of each element is the same on both sides of the equation, reflecting the real-world behavior of chemical reactions.
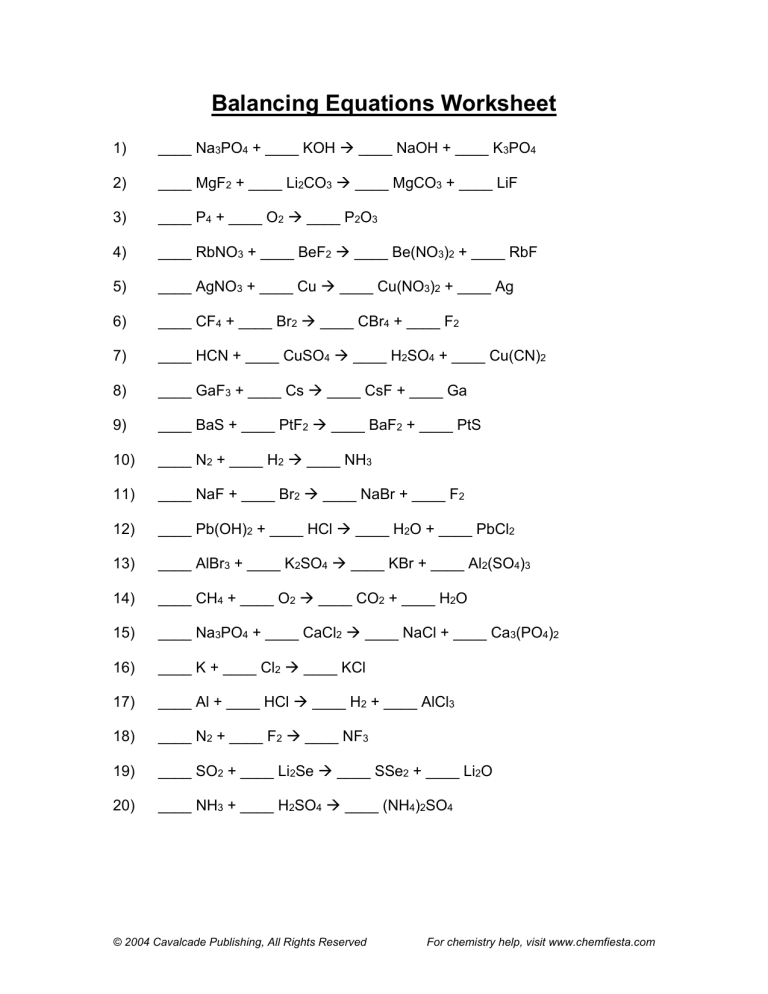
Can I balance a chemical equation by changing subscripts?
+
No, you should never change the subscripts in a chemical formula. Subscripts define the compound’s composition, and altering them would create an entirely different compound. Only coefficients can be changed to balance the equation.
How do I know if I’ve correctly balanced a chemical equation?

+
You’ve correctly balanced an equation when the number of atoms for each element is the same on both sides of the equation, and the coefficients are in their simplest whole-number ratios.
What resources can I use to practice balancing equations?
+
There are numerous online platforms, chemistry apps, and textbooks that provide practice problems for balancing chemical equations. Some resources also offer step-by-step solutions to help guide your learning process.