5 Ways to Master Valence Electrons Easily
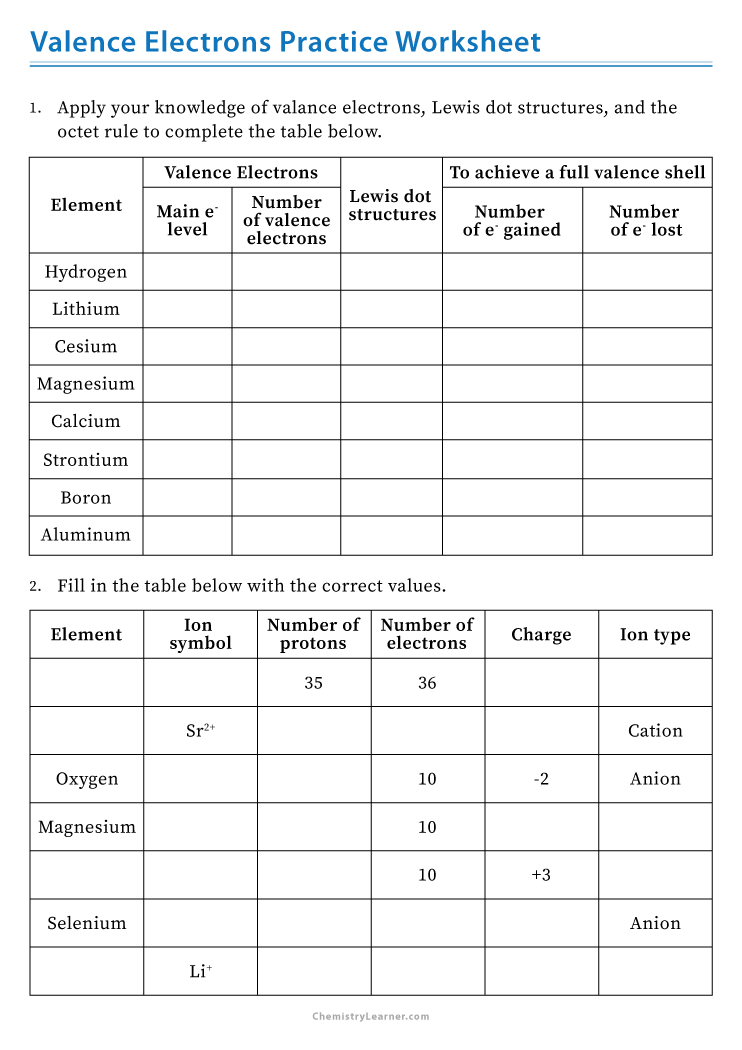
Understanding valence electrons is fundamental in chemistry because these electrons determine an atom's reactivity and bonding behavior. Here are five straightforward strategies to master this key concept:
1. Understanding Electron Configuration
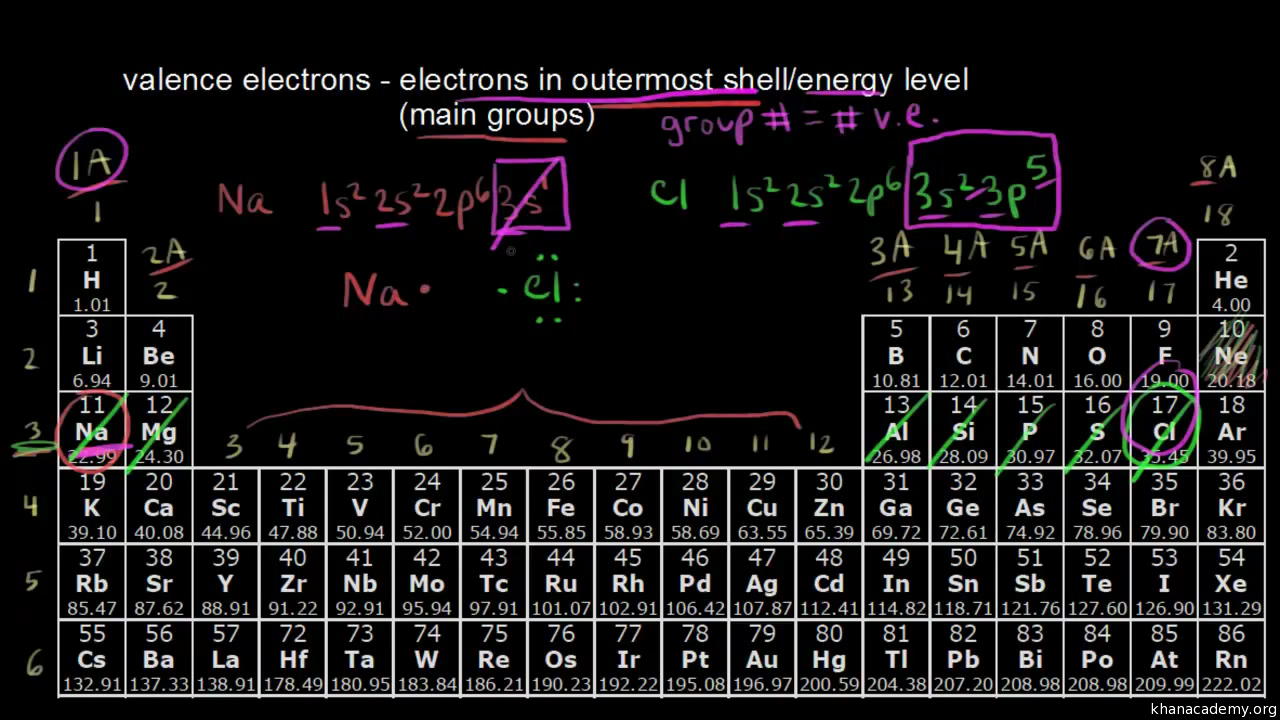
To grasp how valence electrons work, begin with the electron configuration of atoms. This is where each electron’s placement in an atom’s energy levels or shells is noted. Here’s how to approach this:
- Learn the periodic table: Understanding groups (columns) in the periodic table can provide instant clues about valence electrons. For example, elements in the same group have the same number of valence electrons.
- Identify the shells: The outermost shell or highest energy level contains the valence electrons. Typically, for most elements, these are the electrons in the n (principal quantum number) = s, p, or d orbitals.
- Use Aufbau Principle: Electrons fill the lowest available energy levels before moving to higher ones. Remember the sequence 1s, 2s, 2p, 3s, 3p, 4s, 3d, and so forth.
2. Practice with Group Trends
Every element in the same column (group) of the periodic table will share the same number of valence electrons:
| Group | Number of Valence Electrons |
|---|---|
| 1 (Alkali Metals) | 1 |
| 2 (Alkaline Earth Metals) | 2 |
| 13 | 3 |
| 14 | 4 |
| 15 | 5 |
| 16 | 6 |
| 17 (Halogens) | 7 |
| 18 (Noble Gases) | 8 (or 2 for Helium) |
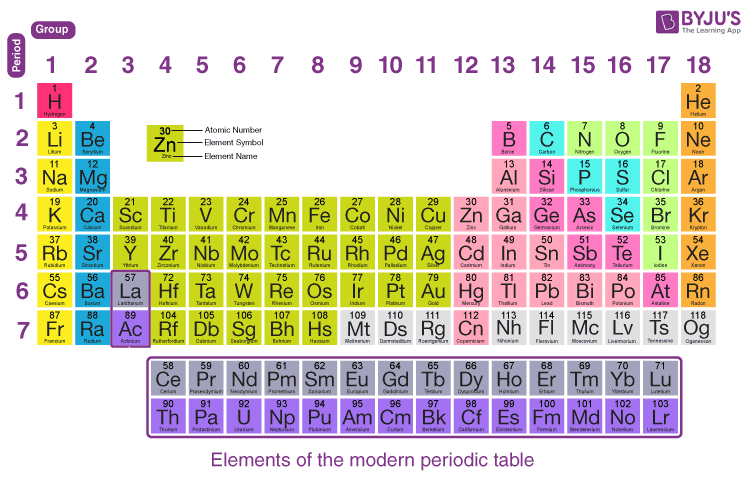
Understanding these trends can make recognizing valence electrons much more manageable.
💡 Note: Transition metals (groups 3-12) can be trickier as their valence electrons often include d orbitals; however, they generally follow the same s and p orbital filling rules for valence counting.
3. Use Dot Structures or Lewis Diagrams
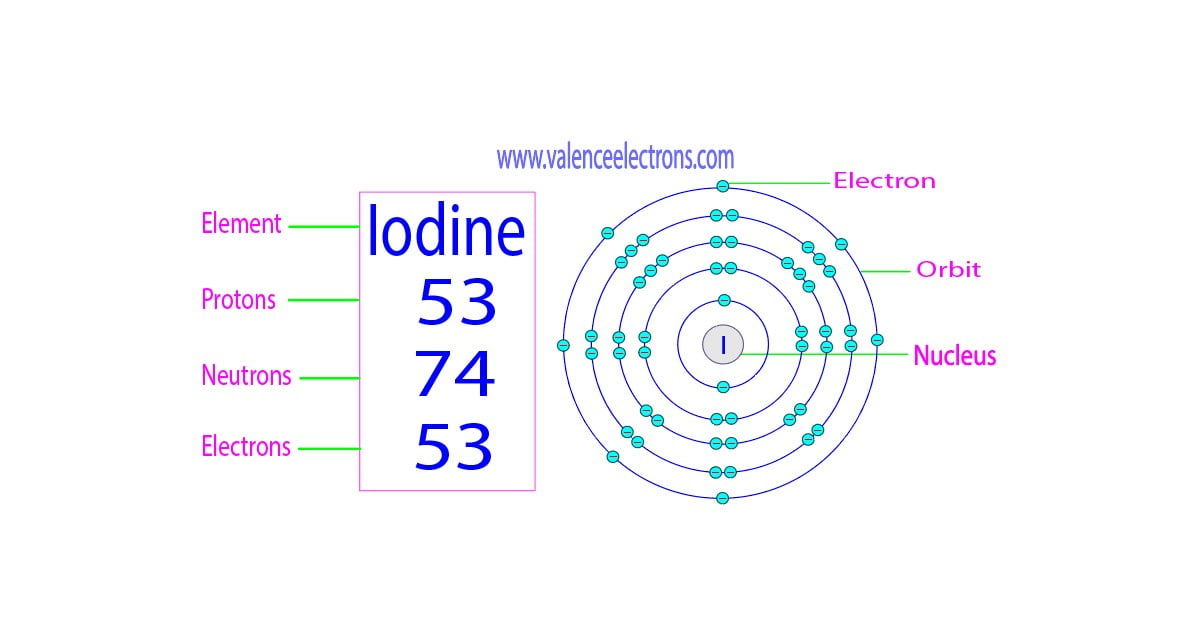
Lewis dot structures offer a visual way to identify valence electrons. Here’s how:
- Draw the symbol: Use the element’s symbol to represent the nucleus and inner shells.
- Add dots: Each dot represents one valence electron. Place up to two dots per side around the symbol, then add the remaining dots if necessary.
These diagrams can help you quickly determine how many valence electrons an atom has, which is crucial for predicting its chemical behavior.
4. Bonding and Electron Transfer
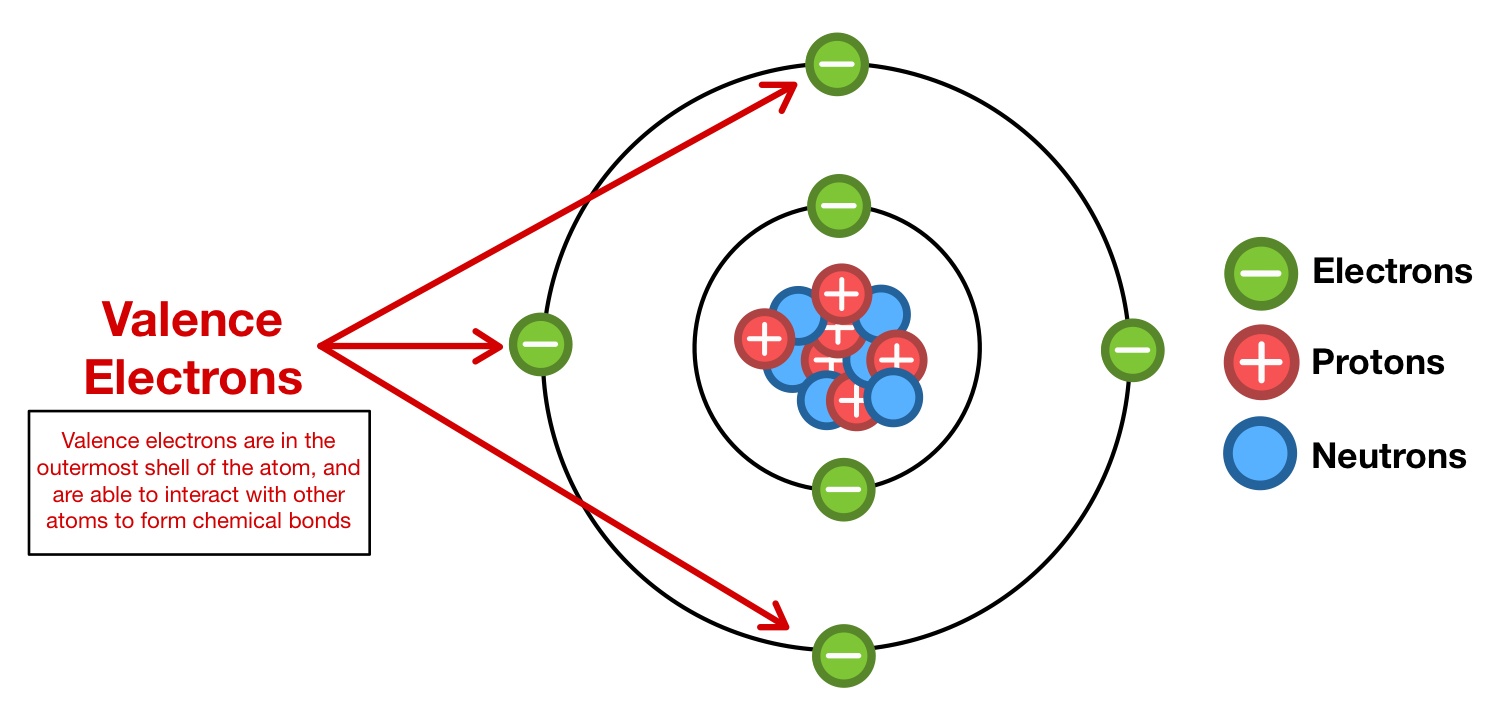
Atoms typically gain, lose, or share electrons to achieve a stable octet or duet configuration:
- Ionic Bonding: Atoms gain or lose electrons to become ions, creating a stable octet. Electronegative atoms like Fluorine will gain electrons, while electropositive ones like Sodium lose electrons.
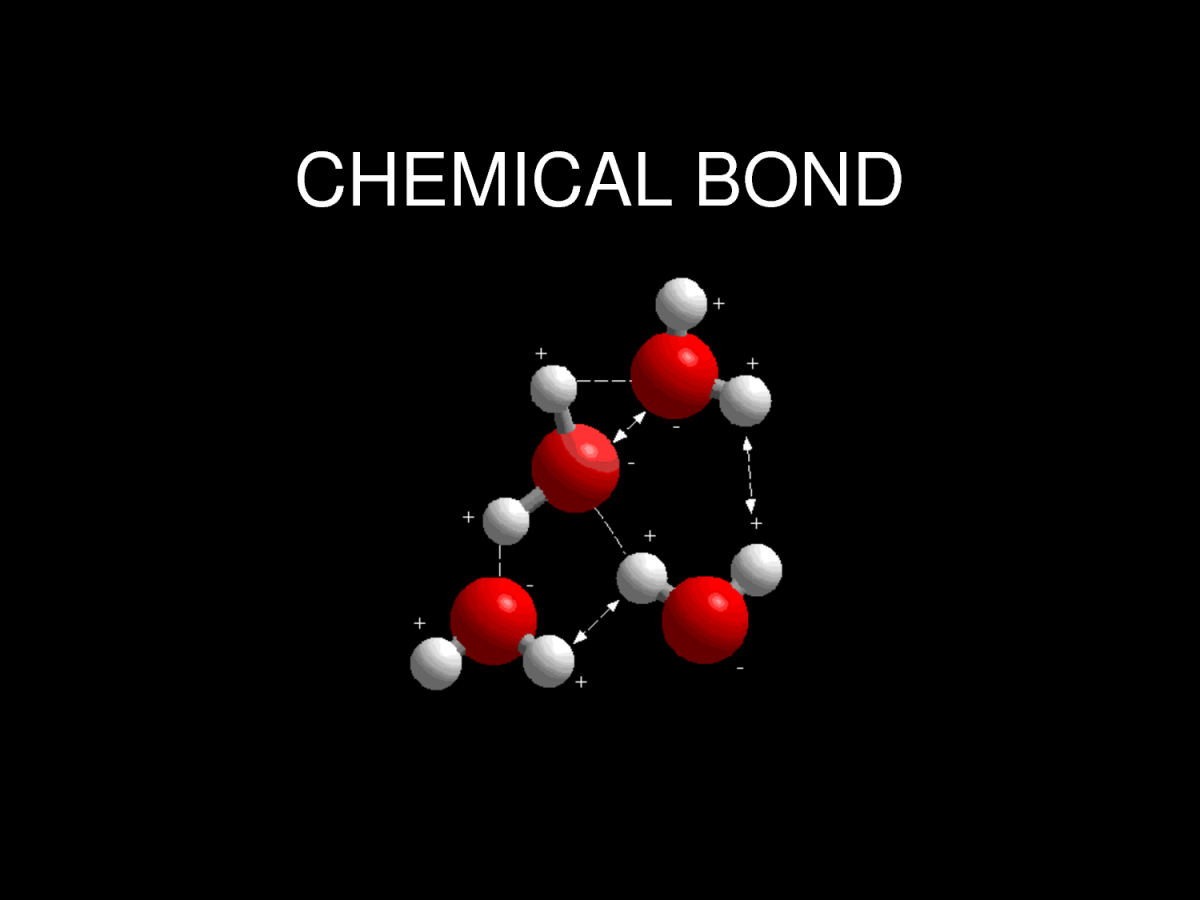
- Covalent Bonding: Atoms share their valence electrons. Molecules like water (H2O) illustrate how hydrogen atoms share electrons with oxygen to fulfill both atoms’ octets.
Understanding these bonding mechanisms helps visualize valence electron behavior in different compounds.
5. Utilize Electron Configurations of Ions
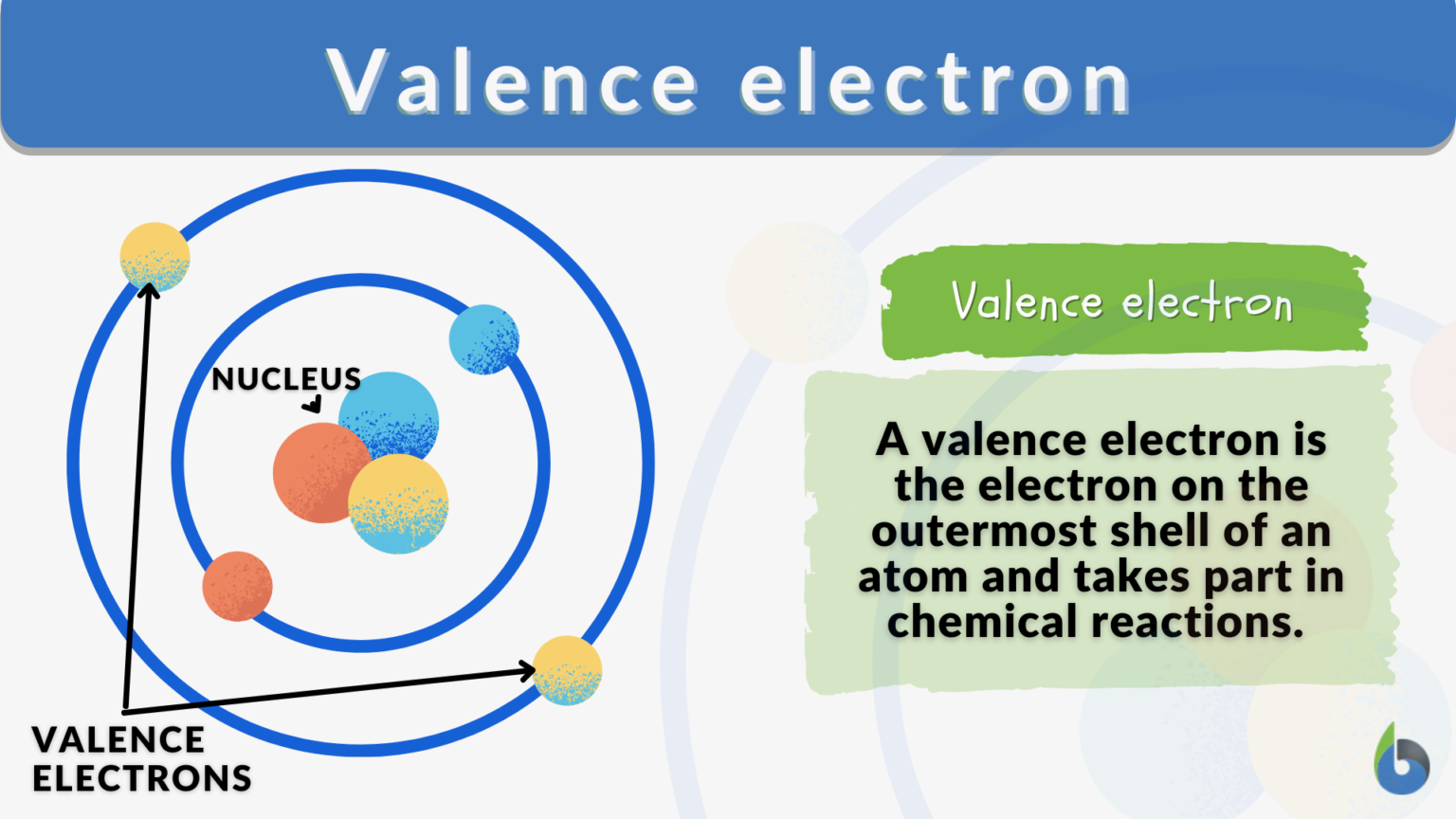
Valence electrons also play a crucial role when atoms form ions. Consider the following:
- Cation Formation: Atoms lose electrons from their outer shell to form positively charged ions (cations). For example, Na+ has one less electron than Na, making it have no electrons in its outer shell (formerly 1s22s22p63s1 becomes 1s22s22p6).
- Anion Formation: Atoms gain electrons to form negatively charged ions (anions). For instance, Cl- has one more electron than neutral chlorine (formerly 1s22s22p63s23p5 becomes 1s22s22p63s23p6).
Remember that transition metals can form multiple types of ions, and their valence electron configurations can differ based on the oxidation state.
To truly understand valence electrons, regular practice and visualizing their behavior through diagrams, configurations, and bonding patterns are essential. The more you delve into these strategies, the more intuitive identifying and predicting valence electron behavior will become. This foundational knowledge not only enhances your chemical understanding but also makes studying chemistry more engaging and easier to comprehend.
What are valence electrons?

+
Valence electrons are the electrons located in the outermost shell of an atom. They determine the atom’s chemical properties, such as how it will react with other atoms or form bonds.
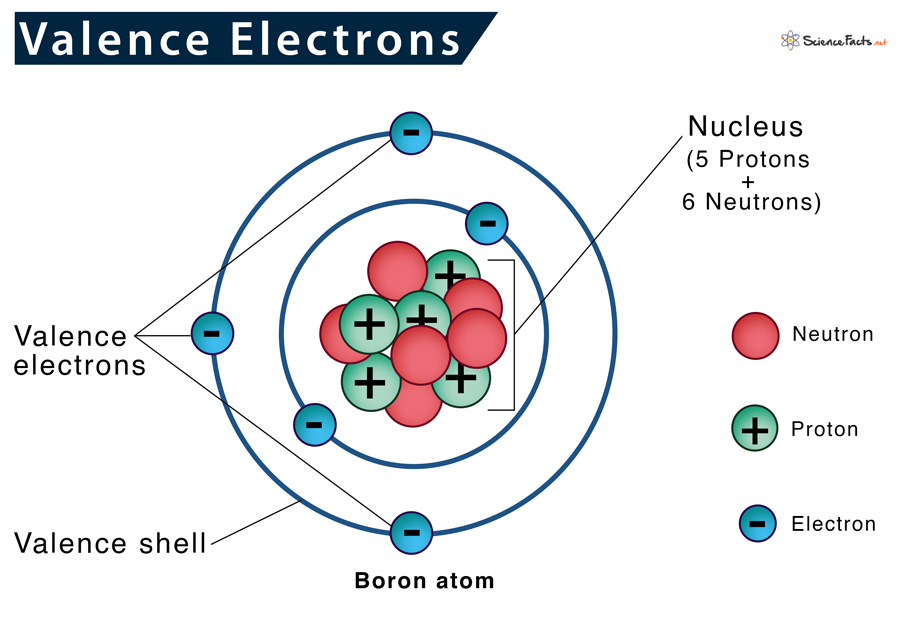
Why do elements in the same group have the same number of valence electrons?
+
Elements in the same group of the periodic table share the same number of valence electrons because they have the same electron configuration in their outermost energy level. This similarity in electron structure leads to similar chemical behavior.
Can transition metals have different numbers of valence electrons?
+
Yes, transition metals can exhibit variable valence electron counts due to the energy levels of their d-orbital electrons, which can participate in bonding in different oxidation states.