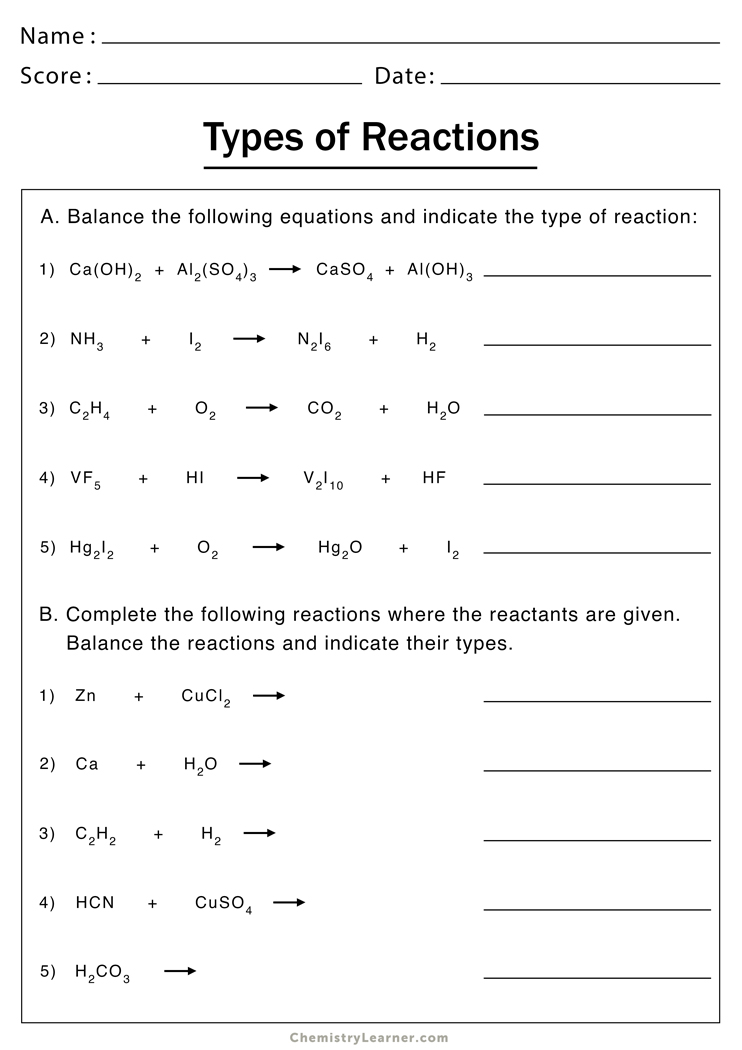
5 Types of Chemical Reactions: Worksheet Answers

If you're navigating through the fascinating and complex world of chemistry, understanding the different types of chemical reactions is fundamental. These reactions are not just lines on a paper but the very processes that shape our world, from the food we digest to the air we breathe. Here, we delve into five primary categories of chemical reactions, providing you with clear explanations and examples to help you master this essential chemistry concept.
Combustion Reactions

A combustion reaction is an exothermic reaction where a substance reacts with oxygen, producing energy in the form of heat and light. Often, these reactions are the basis for fuels and explosions.
- Equation: Hydrocarbon + O₂ → CO₂ + H₂O + Energy
- Example: Methane (natural gas) combustion: CH₄ + 2O₂ → CO₂ + 2H₂O + Heat
🔥 Note: Combustion reactions do not always produce water; if the hydrocarbon lacks hydrogen, you might just get CO₂ or other oxides.
Synthesis Reactions
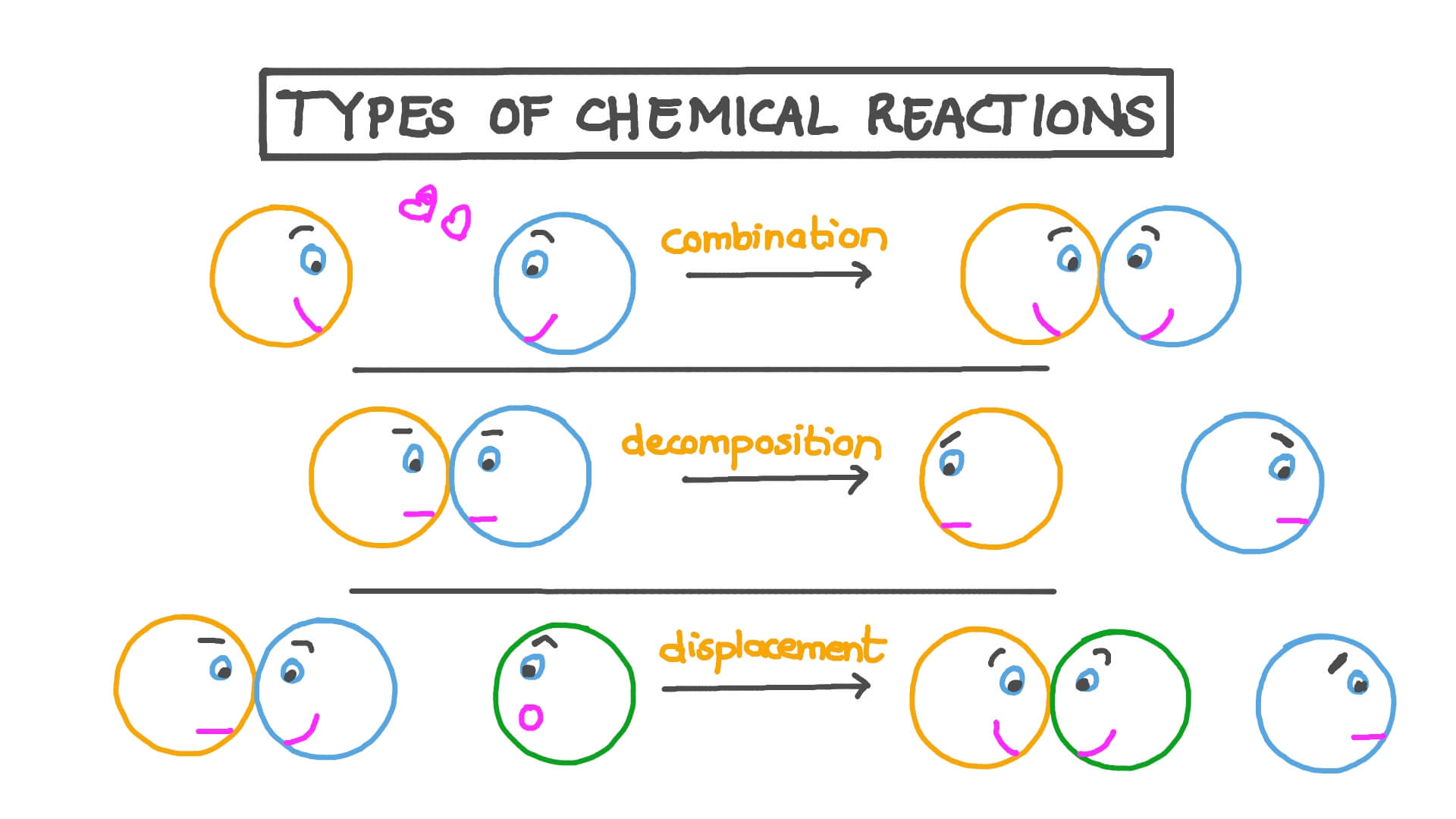
Synthesis, or combination, reactions are when two or more substances combine to form a more complex compound. Here, simplicity converges into complexity.
- Equation: A + B → AB
- Example: Synthesis of water: 2H₂ + O₂ → 2H₂O
Decomposition Reactions

In these reactions, a single compound breaks down into two or more simpler substances. It’s like watching a complex structure disintegrate.
- Equation: AB → A + B
- Example: Thermal decomposition of calcium carbonate: CaCO₃ → CaO + CO₂
Single Replacement Reactions

Single replacement reactions involve one element swapping with another in a compound, creating a new element and compound.
- Equation: A + BC → AC + B (or BA if A is a metal)
- Example: Reaction of zinc with hydrochloric acid: Zn + 2HCl → ZnCl₂ + H₂
Double Replacement Reactions

Here, two compounds switch ions, often forming a solid precipitate or water alongside gases.
- Equation: AB + CD → AD + CB
- Example: Reaction of silver nitrate with sodium chloride: AgNO₃ + NaCl → AgCl(s) + NaNO₃
Chemical reactions are often accompanied by changes in temperature, color, and state of matter, which can serve as indicators of the reaction type. Balancing equations is a crucial step in predicting the outcomes of reactions, ensuring mass conservation.
| Reaction Type | Equation |
|---|---|
| Combustion | Hydrocarbon + O₂ → CO₂ + H₂O + Energy |
| Synthesis | A + B → AB |
| Decomposition | AB → A + B |
| Single Replacement | A + BC → AC + B (or BA) |
| Double Replacement | AB + CD → AD + CB |

By now, you should have a fundamental grasp of these chemical reaction types. Here's your takeaway:
Each reaction type follows a predictable pattern, making it easier to understand complex reactions by breaking them down into these fundamental forms. Combustion, synthesis, decomposition, single replacement, and double replacement reactions cover most chemical transformations in everyday life and industrial processes. When studying chemical reactions, recognize these patterns to predict outcomes, which can be crucial for chemists and students alike. Remember, balancing the chemical equation is key to ensure no atoms are lost or gained, adhering to the law of conservation of mass.
Why is balancing chemical equations important?

+
Balancing chemical equations is crucial to adhere to the law of conservation of mass, which states that the mass of reactants must equal the mass of the products. If an equation isn’t balanced, you might end up with atoms out of thin air or disappearing into nothing!
Can all chemical reactions be categorized under these five types?

+
Most common reactions can be grouped into these five categories, but there are exceptions and more complex reactions that might not fit neatly into one type. For instance, reactions in biochemical processes often require their own classifications due to their intricate nature.
What are some real-world examples of synthesis reactions?

+
Here are a few examples:
- Formation of water from hydrogen and oxygen (2H₂ + O₂ → 2H₂O).
- Photosynthesis: plants combine CO₂ and H₂O to produce glucose (C6H12O6).
- Synthesis of compounds in organic chemistry for drug production.
How does energy play a role in these reactions?

+
Energy is a critical factor in chemical reactions:
- Exothermic reactions release energy, often in the form of heat (e.g., combustion).
- Endothermic reactions absorb energy to proceed (e.g., photosynthesis).
- Activation energy is needed to initiate a reaction, which can be provided through heat, light, or catalysts.
Why do we not always produce water in combustion reactions?
+
Combustion reactions do not always produce water because not all fuels contain hydrogen. For instance, sulfur or carbon can burn in oxygen to produce only carbon dioxide or sulfur dioxide respectively, without water formation.