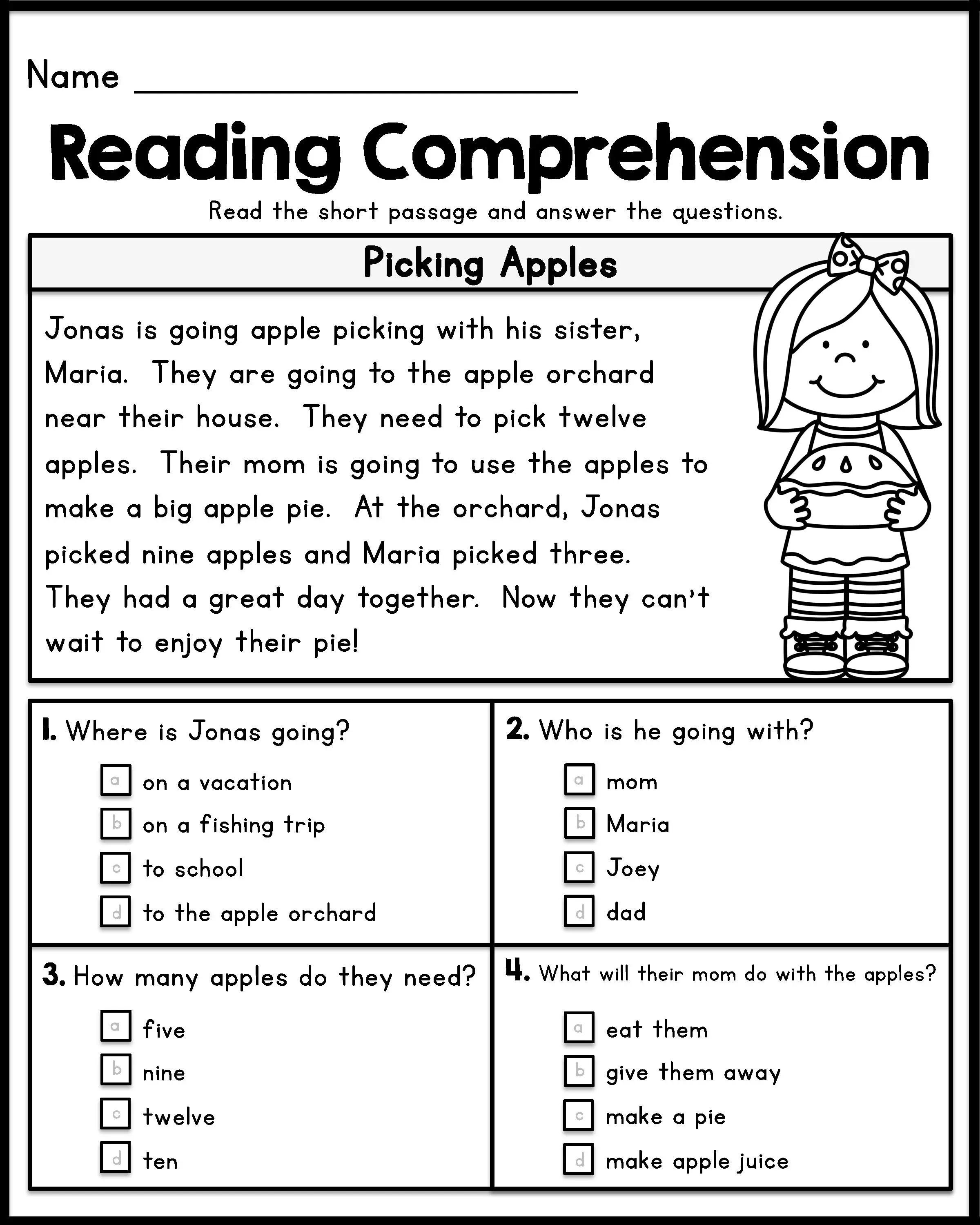
5 Key Answers to Thermochemistry Worksheet Explained

Are you struggling with the fundamental concepts of thermochemistry? Whether you're a student trying to understand the energy changes during chemical reactions or an educator looking for ways to explain these concepts clearly, this blog post will cover five critical answers to common thermochemistry worksheet questions. Let's delve into the world of energy, heat, and reactions with a blend of simplicity and depth, optimizing the content for SEO along the way.
1. What is Enthalpy?

Enthalpy, often denoted as H, is a measure of the total energy of a thermodynamic system. It includes the internal energy, the pressure, and volume of the system, represented mathematically as:
H = U + pV
Here, U is the internal energy, p is the pressure, and V is the volume of the system. Enthalpy is a useful concept in thermodynamics because under constant pressure, the change in enthalpy (ΔH) corresponds to the heat absorbed or released by a system during a reaction. This can be expressed as:
- Endothermic reactions where heat is absorbed have a positive ΔH.
- Exothermic reactions where heat is released have a negative ΔH.
🔥 Note: Enthalpy does not represent the absolute energy of a system but rather the change in energy when a system undergoes a chemical transformation.
2. How Do You Calculate Heat Change (q) in a Reaction?

To calculate the heat change in a reaction, we use the equation:
q = m c ΔT
Where:
- q is the heat absorbed or released.
- m is the mass of the substance.
- c is the specific heat capacity of the substance.
- ΔT is the change in temperature.
This equation is typically used in experiments like calorimetry, where reactions are conducted in a controlled environment. Here’s a practical scenario:
- If a calorimeter contains water and the reaction occurs, you would measure the temperature change of the water. The heat absorbed or released by the reaction can then be determined using the specific heat capacity of water.
Keep in mind that the accuracy of this calculation depends heavily on precise temperature measurements and the correct value for the specific heat capacity of the involved substances.
3. What is Hess’s Law and How Does it Help in Thermochemistry?

Hess’s Law states that the total enthalpy change in a chemical reaction is independent of the number of steps or pathways taken, provided the initial and final conditions are the same. This principle allows chemists to sum up the enthalpies of formation to find the enthalpy change for a complex reaction indirectly.
For instance, if you know:
- Reaction 1: A + B → C, ΔH1
- Reaction 2: C → D, ΔH2
The enthalpy change for the overall reaction (A + B → D) would be:
ΔH1 + ΔH2
🔬 Note: Hess’s Law is particularly useful for reactions that are difficult to conduct directly or when measuring the heat of formation indirectly.
4. Understanding the First Law of Thermodynamics

The First Law of Thermodynamics, also known as the Law of Conservation of Energy, states that energy cannot be created or destroyed, only transferred or transformed from one form to another. In thermochemical terms, this is expressed as:
ΔU = q + w
Where ΔU is the change in internal energy, q is the heat absorbed or released, and w is the work done by or on the system. For many chemical reactions, especially those at constant pressure, work done can often be ignored, and thus:
ΔU ≈ q
Understanding this law is crucial for predicting how energy flows in a system, particularly in terms of:
- Heating or cooling
- Volume change against constant pressure
- Energy transfer through various pathways
5. How to Determine Enthalpy of Reaction from Bond Energies?

The enthalpy of reaction can be estimated by considering the bonds broken and formed during the reaction. Here’s how:
- Bond Breaking: Energy is absorbed to break chemical bonds. The sum of the bond dissociation energies for all broken bonds is considered.
- Bond Forming: Energy is released when new bonds are formed. The sum of the bond formation energies is subtracted.
| Bond | Average Bond Energy (kJ/mol) |
|---|---|
| C-H | 413 |
| O-H | 467 |
| C-O | 358 |
| C=O | 745 |

The total enthalpy change (ΔH) is then:
ΔH = (Sum of Energy of Bonds Broken) - (Sum of Energy of Bonds Formed)
This method provides an approximate value for the enthalpy change of a reaction, particularly useful when direct calorimetric data is not available.
In summary, this exploration into thermochemistry offers a nuanced understanding of energy changes in chemical systems. From understanding enthalpy and heat change calculations to leveraging Hess's Law and bond energies, these key answers provide a foundational knowledge that can help navigate complex chemical processes with confidence. Thermochemistry not only explains why reactions occur but also guides scientists in engineering reactions for specific industrial applications, thereby underlining its significance in both academic and practical chemistry.
Why is enthalpy used rather than internal energy in chemical reactions?

+
Enthalpy is often used instead of internal energy in chemical reactions at constant pressure because it accounts for changes in both internal energy and the work done by or against the system’s surroundings. This makes it more relevant to the conditions under which most chemical reactions occur.
Can you explain what specific heat capacity is?

+
Specific heat capacity is the amount of heat required to raise the temperature of one unit mass of a substance by one degree Celsius (or Kelvin). Different substances have different capacities to absorb heat, which affects the amount of energy needed to change their temperature.
What’s the difference between endothermic and exothermic reactions?

+
Endothermic reactions absorb heat from their surroundings, resulting in a positive change in enthalpy (ΔH > 0). Exothermic reactions release heat into the surroundings, leading to a negative change in enthalpy (ΔH < 0).