5 Key Insights: Atom Structure Worksheet Answers

Understanding the basic building blocks of matter is an essential topic in the education of budding scientists, students, and anyone curious about the natural world. Delving into the specifics of atomic structure can open up a universe of fascination, and answering worksheet questions helps consolidate this knowledge. Here are five key insights into atom structure worksheet answers, designed to guide you through the complexities of atoms.
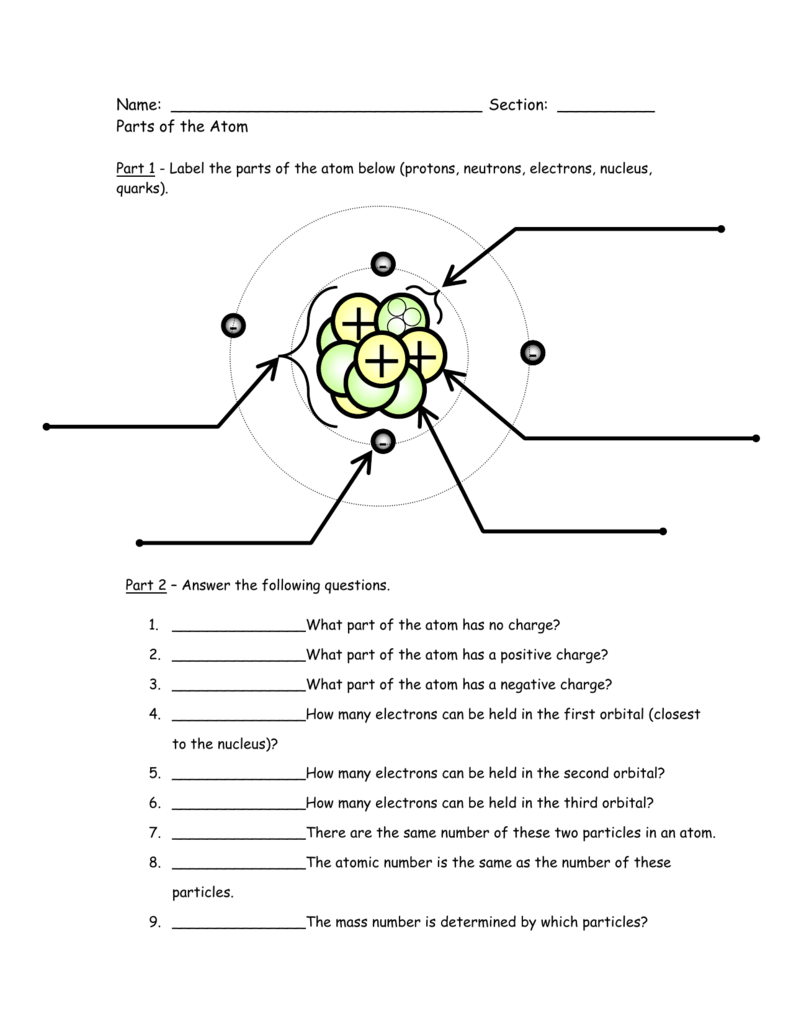
1. Protons, Neutrons, and Electrons: The Core Elements

Atoms are made up of three primary subatomic particles: protons, neutrons, and electrons. Here’s what each particle contributes to an atom:
- Protons: Positively charged, found in the nucleus, and determine the atomic number.
- Neutrons: Neutral particles located in the nucleus, contributing to the atom’s mass.
- Electrons: Negatively charged, orbiting the nucleus in shells, and involved in chemical bonding.
⚠️ Note: The number of protons is equal to the atomic number, which uniquely identifies each element.
2. Atomic Number and Mass Number

Understanding atomic number and mass number is crucial for deciphering an element’s identity:
- Atomic Number: This is the number of protons in the nucleus, dictating the element’s position in the periodic table.
- Mass Number: The sum of protons and neutrons in an atom’s nucleus.
To calculate the number of neutrons, you subtract the atomic number from the mass number:
Number of Neutrons = Mass Number - Atomic Number
3. Electron Shells and Configuration

The arrangement of electrons in their shells around the nucleus impacts the atom’s chemical properties. Here’s a brief overview:
- Electrons fill shells in order of increasing energy.
- The first shell can hold a maximum of 2 electrons, the second shell 8, and so on.
The electron configuration describes how these electrons are arranged:
| Element | Electron Configuration |
|---|---|
| Hydrogen | 1s1 |
| Helium | 1s2 |
| Lithium | 1s2 2s1 |
The octet rule states that atoms are stable when their outermost shell has 8 electrons, but this doesn’t apply to all elements.
4. Isotopes

Atoms of the same element can have different numbers of neutrons, known as isotopes. This variation doesn’t affect chemical behavior but can alter physical properties:
- Stable isotopes can occur naturally.
- Some isotopes are radioactive, meaning they undergo decay to achieve stability.
5. Valency and Bonding

An atom’s ability to form chemical bonds, or its valency, is determined by the number of electrons in its outermost shell. Here’s what you should know:
- Elements tend to form bonds to achieve a full outer shell (following the octet rule).
- Bonds can be ionic (transfer of electrons) or covalent (sharing of electrons).
Understanding atom structure worksheet answers requires knowing these fundamental principles of atomic structure. By exploring these concepts, students and enthusiasts alike can grasp how atoms interact, bond, and contribute to the physical world we observe.
What is the significance of atomic number in an atom?

+
The atomic number signifies the number of protons in an atom’s nucleus, which uniquely identifies the element. It also determines the element’s position in the periodic table.
How can an isotope be identified?

+
An isotope can be identified by its mass number, which differs from the element’s standard atomic weight due to variations in neutron count.
Why do electron configurations matter?

+
Electron configurations provide insights into an atom’s chemical behavior, stability, and reactivity by indicating how electrons are arranged in shells.
Can the number of neutrons change the chemical properties of an atom?

+
While the number of neutrons can alter an atom’s physical properties (like mass), it does not change its chemical behavior, which is determined by electron configuration.



