Protein Structure Worksheet Answers: Key Guide for Learning
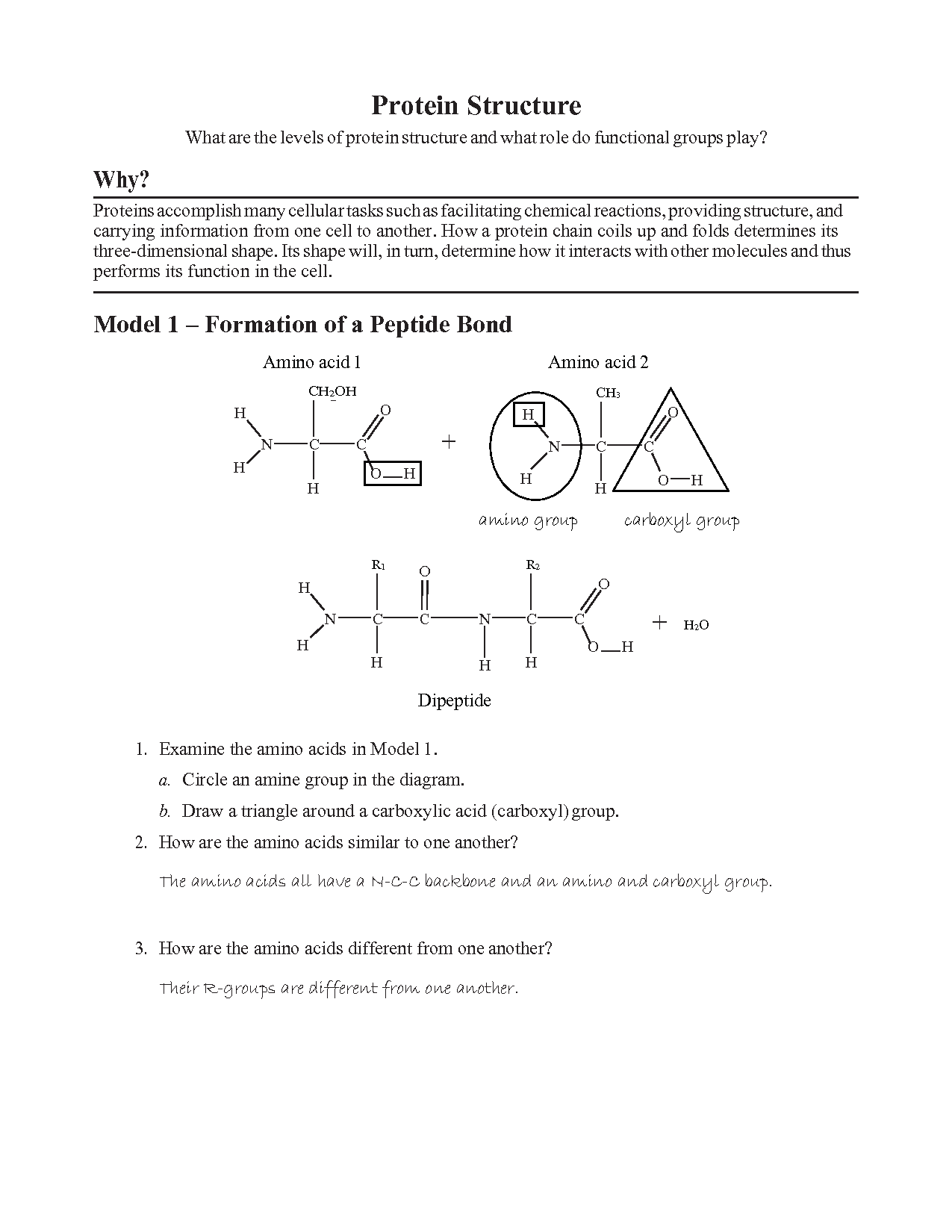
The study of protein structure is fundamental in understanding the biochemical reactions and functions that occur within living organisms. Proteins are complex molecules made up of amino acids, and their structure determines their functionality. This guide will take you through the essential components of protein structure and provide answers to common questions found in protein structure worksheets, aiding in your learning journey.
Primary Structure

The primary structure of a protein refers to the linear sequence of amino acids in its polypeptide chain. Here are some key points:
- Each amino acid is linked to another through peptide bonds.
- The sequence is crucial because it dictates the folding pattern which ultimately leads to the protein’s functional shape.
- Genetic code directly translates into the primary structure through the process of translation.
Here’s an example of how primary structure might be represented:
| Position | Amino Acid |
|---|---|
| 1 | Glycine |
| 2 | Alanine |
| 3 | Serine |

🔍 Note: Alterations in primary structure can lead to changes in protein function or even cause diseases like sickle cell anemia due to the single amino acid mutation in the hemoglobin protein.
Secondary Structure


The secondary structure involves the local folding of the polypeptide chain into specific patterns:
- Alpha-helices (α-helices): Tight coils like a coiled spring, stabilized by hydrogen bonds between the amide hydrogens and carbonyl oxygens of the peptide backbone.
- Beta-sheets (β-sheets): Extended strands of protein that can either run parallel or anti-parallel, connected by hydrogen bonds between adjacent strands.
- Other structures like turns and loops provide flexibility to the protein.
The stability and characteristics of these structures are essential for protein functionality, such as:
- Flexibility to interact with other molecules.
- Formation of enzymatic active sites.
- Proper interaction with nucleic acids or lipids.
Tertiary Structure


The tertiary structure describes the overall three-dimensional shape of a single protein molecule, resulting from various types of interactions between the amino acid side chains:
- Hydrophobic interactions: Non-polar side chains cluster together away from water.
- Hydrogen bonds: Between polar side chains or between side chains and the backbone.
- Ionic bonds (salt bridges): Between positively and negatively charged side chains.
- Disulfide bridges: Formed by covalent bonding between cysteine residues.
- Van der Waals forces: Weak attractions between non-polar groups.
This complex interplay of forces gives proteins their unique shape, which is critical for:
- Enzyme activity and substrate binding.
- Ligand binding in receptors.
- Transmembrane protein function in cell membranes.
🔬 Note: The shape of proteins can be altered by factors like pH, temperature, or the presence of certain chemicals, leading to denaturation where proteins lose their functionality.
Quaternary Structure


Not all proteins have a quaternary structure, but for those that do, it refers to the arrangement of multiple protein subunits to form a functional protein complex. Here are the key points:
- The subunits can be identical (homo-oligomers) or different (hetero-oligomers).
- Interactions between subunits are similar to those in tertiary structure but include additional interface interactions.
- Important for cooperative effects in enzymes or for structural integrity.
Functions of Proteins

Proteins perform various functions due to their unique structures:
- Enzymatic activity: Catalyzing biochemical reactions.
- Structural proteins: Providing support or shape like collagen in the extracellular matrix.
- Transport: Hemoglobin transports oxygen.
- Immune responses: Antibodies and immune receptors.
- Cell signaling: Receptors and hormones.
By understanding the structure of proteins, we gain insights into how they work at a molecular level, their interactions, and their malfunction when the structure is disrupted.
In Conclusion

Exploring the nuances of protein structure not only illuminates the elegance of biological systems but also provides a foundation for advances in various scientific fields. From the straightforward linear arrangement of amino acids in the primary structure to the complex assembly of quaternary structure, each level of protein folding contributes to the vast array of functions that proteins perform. The keys to unlocking the mysteries of life’s processes are often found in the intricate architecture of these biomolecules. As students, researchers, or curious minds, delving into protein structure through this guide will enhance your understanding of one of the most fundamental aspects of life science.
What determines the primary structure of proteins?

+
The primary structure of proteins is determined by the genetic code in the DNA. The sequence of nucleotides in the gene dictates the order of amino acids during protein synthesis.
Why do proteins have secondary structures?

+
Secondary structures form due to local folding patterns driven by hydrogen bonding between the peptide backbone’s amide hydrogens and carbonyl oxygens. These structures are essential for protein stability and function.
How do changes in protein structure affect its function?

+
Changes in protein structure, particularly through mutations or environmental factors, can alter or completely disrupt the protein’s ability to function properly, often leading to disease or dysfunction.
What is the role of disulfide bridges?

+
Disulfide bridges are covalent bonds between sulfur atoms in cysteine residues, stabilizing the protein’s tertiary and quaternary structure by locking certain parts of the protein in place.