Discover Water's Wonders: Properties Worksheet Answers Revealed
The beauty and complexity of water are often overlooked, but its role in our daily lives is unparalleled. This post will dive deep into the properties of water, exploring why it's essential and how understanding these properties can enhance our appreciation of this remarkable substance. From its ability to dissolve a wide array of substances to its unique physical attributes, we'll uncover all you need to know about water's wonders.
Exploring the Basic Properties of Water

Water's properties are intriguing, making it not just the source of life but a substance with extraordinary characteristics. Here's a basic rundown:
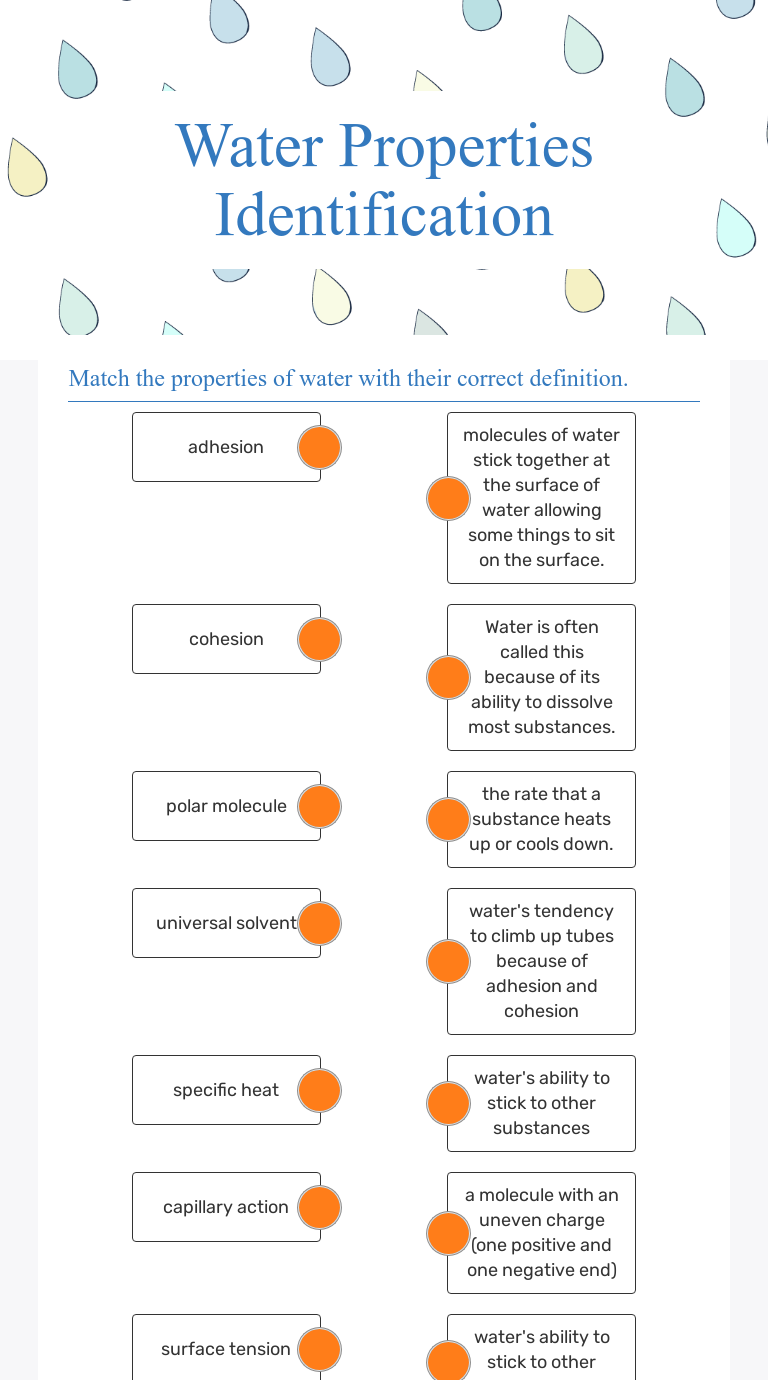
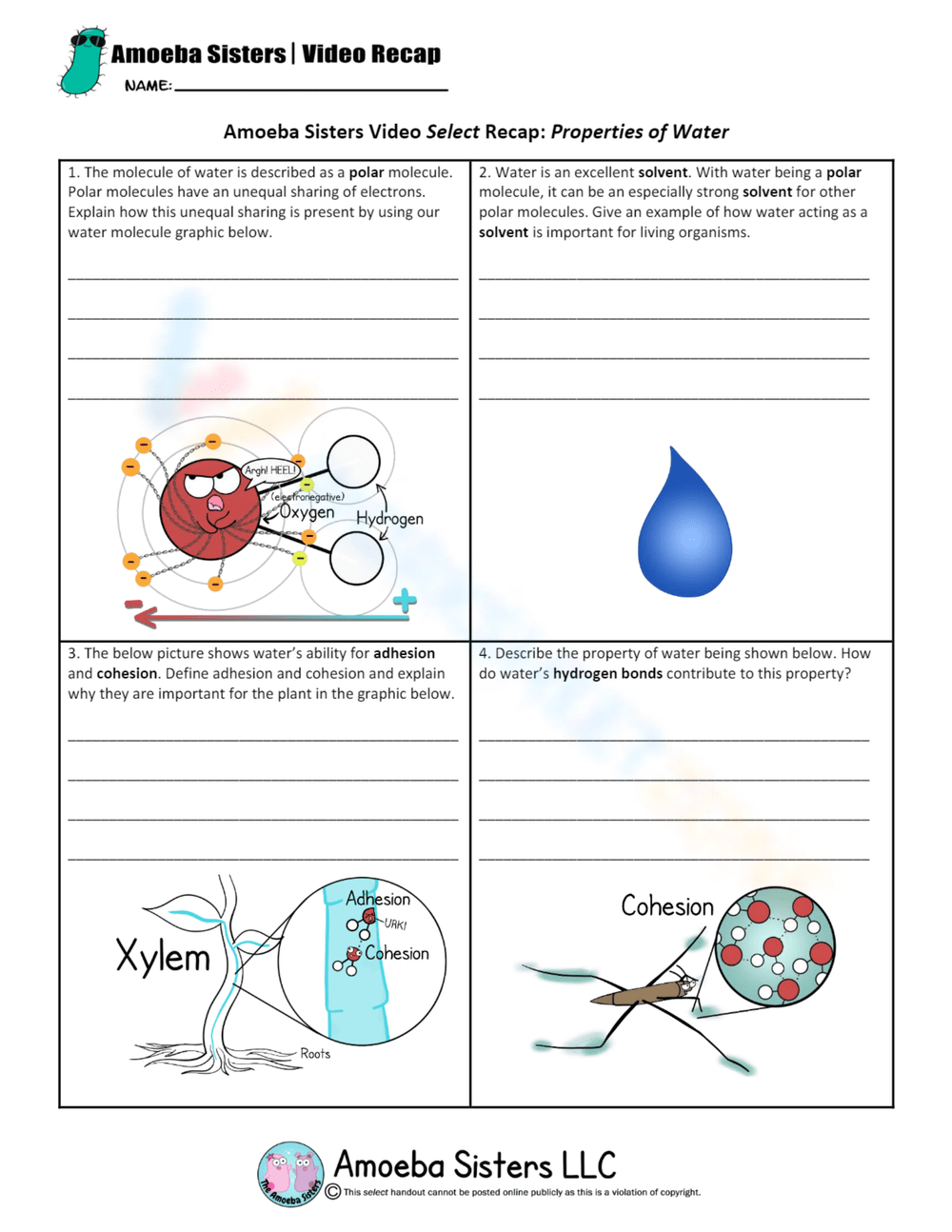
- Polarity: Water is a polar molecule due to the difference in electronegativity between oxygen and hydrogen atoms, which results in a slightly positive charge on hydrogen atoms and a slightly negative charge on the oxygen atom.
- Hydrogen Bonding: This polarity leads to hydrogen bonding, giving water its unique properties like high boiling point, surface tension, and cohesion.
- Heat Capacity: Water has a high specific heat capacity, meaning it can absorb or release a lot of heat with relatively little change in temperature.
- Density: Water is denser than ice, which is unusual and explains why ice floats in liquid water.
The Polarity of Water


Water molecules have a bent structure due to the two lone pairs of electrons on the oxygen atom, which forces the hydrogen atoms to be 104.45 degrees apart. This bent structure creates partial charges, making water an excellent solvent and reactive agent.
💡 Note: Water’s polarity allows it to dissolve a variety of ionic compounds, which is why it’s often called the universal solvent.
Hydrogen Bonding in Water


Hydrogen bonds give water its strength and structure. These bonds form between the hydrogen atom of one water molecule and the oxygen atom of another, creating a vast network of connections within liquid water.
🔬 Note: The hydrogen bonding network in water is so extensive that it’s considered a continuous medium, allowing for water’s remarkable physical properties.
The Heat Capacity of Water

Water’s high heat capacity means it can absorb or release a large amount of heat without significantly changing its temperature. This property is crucial for regulating the Earth’s temperature, moderating climate, and supporting life by stabilizing temperatures in various ecosystems.
🌍 Note: Due to water’s heat capacity, aquatic environments do not experience the same drastic temperature fluctuations as terrestrial environments.
Density Anomalies of Water


The density anomaly of water, where its solid form (ice) is less dense than its liquid form, is due to the open hexagonal structure formed by hydrogen bonds in ice. This unusual characteristic allows ice to float, insulating the water below and affecting climate and aquatic life.
❄️ Note: This property of water is crucial for life in colder climates, as ice acts as a thermal blanket for the water beneath it.
The Universal Solvent: Solubility and Water

Water's ability to dissolve a wide array of substances stems from its polarity and hydrogen bonding. Here’s how it works:
- Ionic Dissolution: Ionic compounds like salts dissolve in water because the polar water molecules surround and separate the ions.
- Polar Compounds: Polar molecules, such as sugars, also dissolve due to their attraction to water's partial charges.
- Hydrogen Bonding: Water's hydrogen bonds facilitate the dissolution of substances like alcohols and acids.
Here's a basic representation of water's solvent abilities:
| Substance | Solubility | Reason |
|---|---|---|
| Sugar (C12H22O11) | Very Soluble | Polar and forms hydrogen bonds with water |
| Table Salt (NaCl) | Very Soluble | Ionic compound, ions are separated by water molecules |
| Oil | Insoluble | Non-polar, unable to form effective bonds with water |
The Importance of Water in Biological Systems

Water's role in biology is multifaceted:
- Transport Medium: It acts as the primary medium for nutrient transport in organisms.
- Temperature Regulation: Its high heat capacity helps in thermoregulation in living beings.
- Solvent for Biochemical Reactions: Many biochemical reactions take place in an aqueous environment, facilitated by water's properties.
Water as a Transport Medium

From the human blood circulation system to plant xylem and phloem, water’s ability to dissolve and transport nutrients and waste is crucial for life processes. The flow of sap in trees, for instance, relies on water’s cohesion and adhesion to defy gravity.
Water and Temperature Regulation

The heat capacity of water allows it to absorb or release large amounts of energy, helping organisms maintain a stable internal environment. This is vital for all living organisms, from mammals sweating to cool down to plants controlling their hydration levels for temperature regulation.
Water’s Role in Biochemical Reactions

Biochemical processes require water as a medium where reactants can interact freely, allowing enzymes to catalyze reactions efficiently. The hydrogen bonding network of water helps stabilize the tertiary structure of proteins, crucial for their function.
Water and Environmental Impact

Water's properties also have significant environmental implications:
- Climate Regulation: Bodies of water influence local climates due to their heat capacity and evaporation processes.
- Soil Retention: Its ability to penetrate and hold soil particles together is essential for plant growth.
- Weathering and Erosion: Water's movement through the environment shapes landscapes through weathering and erosion.
In the final part of our journey through water's wonders, we've explored how its unique properties not only affect our physical and biological world but also contribute to the Earth's environment. Understanding these characteristics underscores the importance of water conservation and the need to protect our water resources. This comprehensive look at water's physical, chemical, and environmental roles shows us why it is truly a substance of wonder, essential for life, and a topic worthy of our deep appreciation and respect.
Why is water known as the universal solvent?

+
Water is known as the universal solvent because it can dissolve a wider range of substances than any other liquid due to its polarity, which allows it to interact with both polar and ionic compounds effectively.
How does water’s heat capacity affect aquatic ecosystems?

+
Water’s high specific heat capacity means it can absorb or release a lot of heat with a small change in temperature. This helps stabilize the temperatures in aquatic ecosystems, allowing for more consistent conditions conducive to life.
What environmental consequences arise from water’s density anomalies?

+
Water’s density anomaly where ice floats on liquid water has profound environmental impacts. It insulates bodies of water from freezing completely, which supports aquatic life by maintaining habitats even in cold climates.