Chemistry Gas Laws Worksheet Answers: Simplified Guide

Understanding the principles of gas laws is fundamental for students studying chemistry, physics, and related fields. These laws provide insights into how gases behave under various conditions of pressure, volume, and temperature. This guide simplifies the complexities of these laws by focusing on Chemistry Gas Laws Worksheet Answers, offering clear explanations and solutions that students often encounter in their studies.
Why Study Gas Laws?

Gas laws are crucial because they:
- Predict gas behavior under different conditions.
- Form the basis for more advanced topics like thermodynamics and kinetics.
- Have practical applications in fields such as engineering, meteorology, and medicine.
The Three Key Gas Laws

Boyle's Law
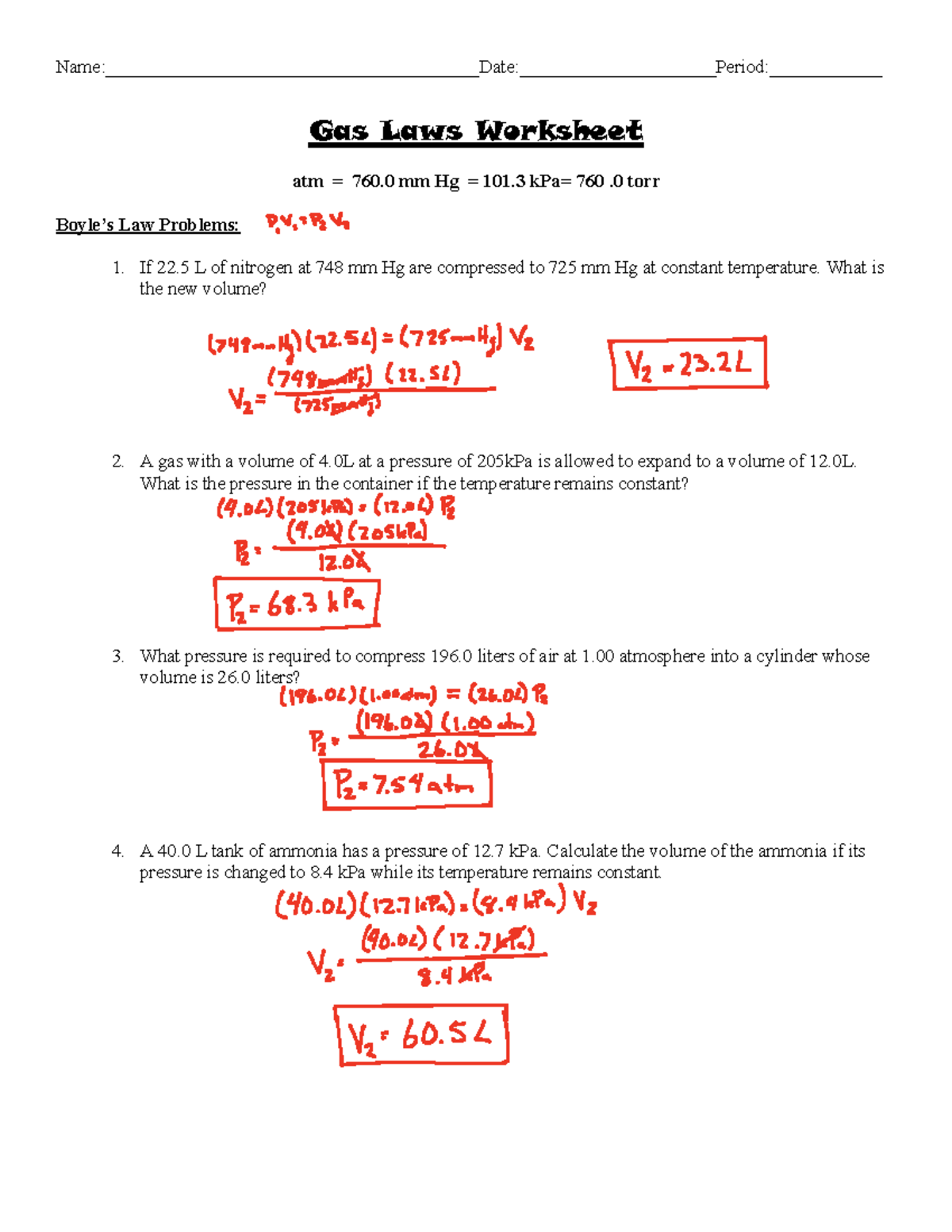
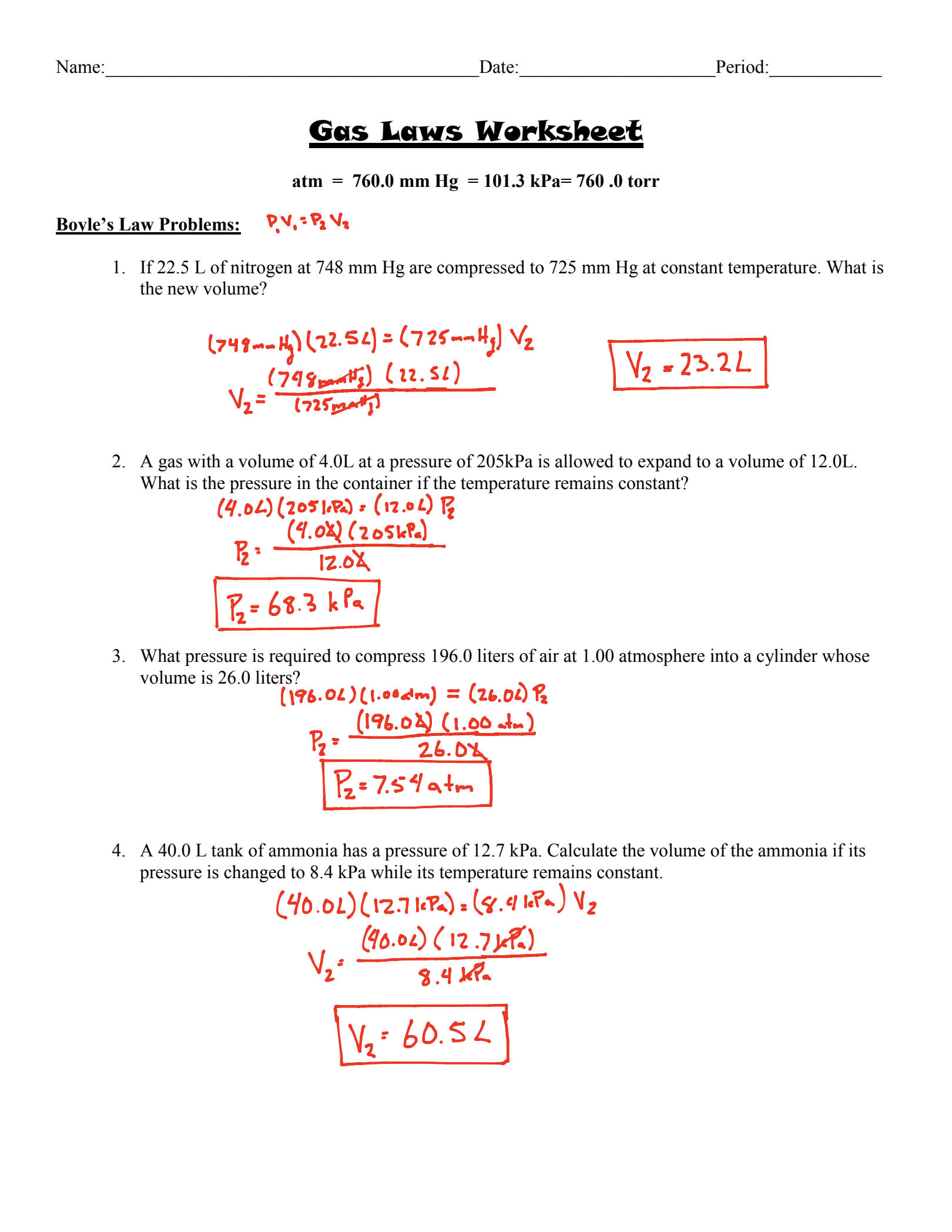
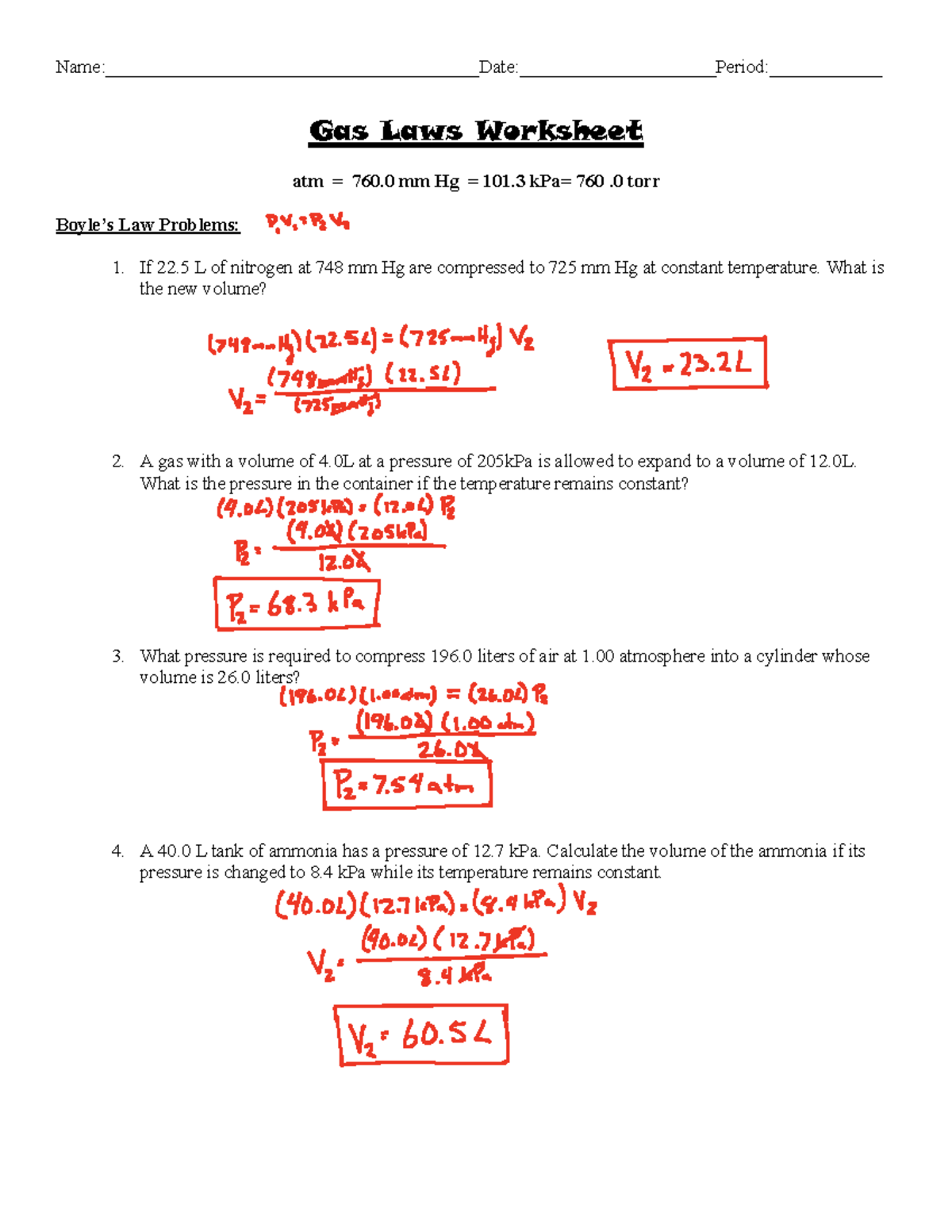
Boyle's Law states that the pressure of a gas decreases as the volume increases when temperature is held constant. Mathematically, this relationship can be expressed as:
[ P_1V_1 = P_2V_2 ]
Example Problem:
If a gas has an initial pressure of 2 atm and a volume of 3 liters, what will its pressure be if the volume is increased to 6 liters?
Solution:
( P_1 = 2 \text{ atm} ), ( V_1 = 3 \text{ liters} ), ( V_2 = 6 \text{ liters} )
( P_2 = \frac{P_1V_1}{V_2} = \frac{2 \times 3}{6} = 1 \text{ atm} )
⚗️ Note: Always check if the temperature remains constant as Boyle’s Law only applies to isothermal processes.
Charles' Law

Charles' Law describes how the volume of a gas expands when heated and contracts when cooled, provided the pressure is constant. The law is:
[ \frac{V_1}{T_1} = \frac{V_2}{T_2} ]
Example Problem:
A balloon has a volume of 500 mL at 273 K. What will its volume be at 373 K?
Solution:
( V_1 = 500 \text{ mL} ), ( T_1 = 273 \text{ K} ), ( T_2 = 373 \text{ K} )
( V_2 = \frac{V_1T_2}{T_1} = \frac{500 \times 373}{273} ≈ 684.7 \text{ mL} )
Gay-Lussac's Law
Gay-Lussac's Law deals with the relationship between pressure and temperature of a gas, with volume being constant:
[ \frac{P_1}{T_1} = \frac{P_2}{T_2} ]
Example Problem:
The pressure of a gas is 1.5 atm at 300 K. What is the pressure if the temperature increases to 450 K?
Solution:
( P_1 = 1.5 \text{ atm} ), ( T_1 = 300 \text{ K} ), ( T_2 = 450 \text{ K} )
( P_2 = \frac{P_1T_2}{T_1} = \frac{1.5 \times 450}{300} = 2.25 \text{ atm} )
Combined Gas Law

The combined gas law integrates Boyle's, Charles', and Gay-Lussac's laws into one comprehensive equation:
[ \frac{P_1V_1}{T_1} = \frac{P_2V_2}{T_2} ]
This equation is particularly useful when changes in more than one parameter (pressure, volume, or temperature) are involved. Here’s a table summarizing how each law relates:
| Law | Fixed Parameter | Equation |
|---|---|---|
| Boyle's Law | Temperature | \[ P_1V_1 = P_2V_2 \] |
| Charles' Law | Pressure | \[ \frac{V_1}{T_1} = \frac{V_2}{T_2} \] |
| Gay-Lussac's Law | Volume | \[ \frac{P_1}{T_1} = \frac{P_2}{T_2} \] |
| Combined Gas Law | None | \[ \frac{P_1V_1}{T_1} = \frac{P_2V_2}{T_2} \] |

Key Takeaways
- Gas laws explain how gases respond to changes in temperature, pressure, and volume.
- Each law focuses on the effect of one or two variables while keeping the others constant.
- The combined gas law provides a holistic approach for when all variables might change.
- Understanding these laws is crucial for calculations in chemical reactions, climate science, and engineering applications.
When studying these laws, ensure to note:
- The units of measurement (pressure in atmospheres, volume in liters, and temperature in Kelvin).
- That all measurements should be made under ideal conditions for the laws to be accurately applied.
- Theoretical understanding is complemented by practical applications in real-world scenarios.
This guide provides answers to common problems found in chemistry gas laws worksheets, helping students to develop a better grasp on how gases behave. Whether preparing for exams or understanding the world around us, the principles of gas laws offer a foundation that is both fascinating and applicable.
How do I remember all these gas laws?

+
One useful mnemonic to remember these laws is: BV (Boyle’s law deals with volume), CT (Charles’ law deals with temperature), and GT (Gay-Lussac’s law deals with temperature). Also, focus on understanding the relationships rather than just memorizing formulas.
Can these gas laws be applied to real gases?

+
While these laws provide a good approximation for how gases behave, real gases deviate from ideal behavior under high pressure or low temperature conditions. However, for most educational and moderate real-world applications, they are still quite accurate.
What are the practical applications of these gas laws?

+
They are applied in:
- Aerospace to calculate lift, thrust, and fuel requirements.
- Medicine for respiratory devices and gas exchange in lungs.
- Engineering for pressure, volume, and temperature controls in machinery.
- Chemistry for reactions where gases are involved or produced.
Related Terms:
- Gas Laws worksheet middle school


