5 Ways to Differentiate Substances from Mixtures

Understanding the difference between substances and mixtures is fundamental in chemistry, impacting everything from industrial processes to everyday decisions in cooking. Both substances and mixtures play critical roles in our daily lives, but they exhibit distinct characteristics that define their behavior, properties, and uses. In this comprehensive guide, we will explore five key methods to distinguish substances from mixtures, providing insights that are valuable for students, scientists, and the generally curious alike.
1. Homogeneity and Heterogeneity
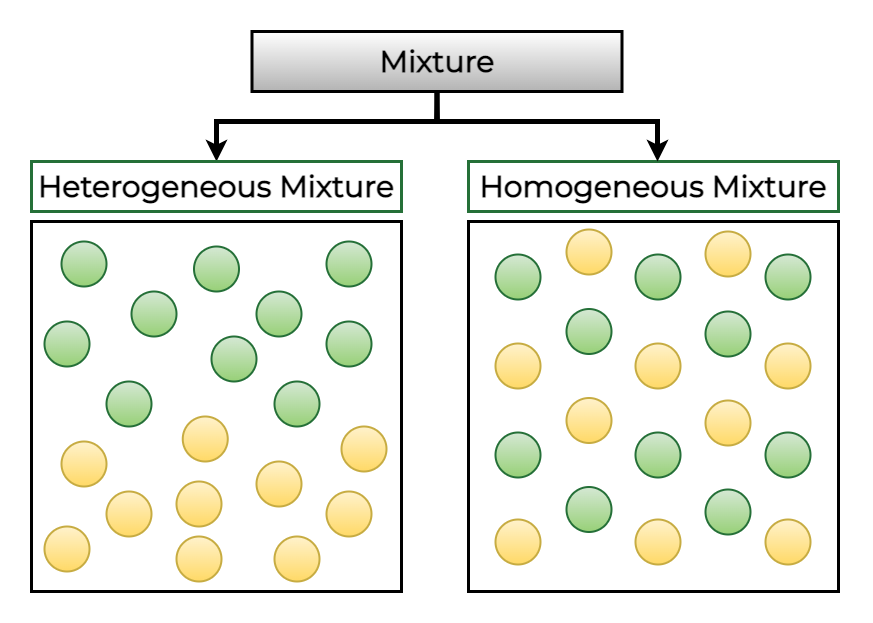
A fundamental difference between substances and mixtures lies in their uniformity:
- Substances are homogeneous, meaning they have a uniform composition throughout. Any sample of a substance will have the same properties.
- Mixtures, on the other hand, can be either homogeneous (solutions like saltwater) or heterogeneous, where different parts of the mixture have different compositions (e.g., trail mix).
Identifying Homogeneity:

- Look at the sample under a microscope or use a magnifying glass for larger particles.
- Observe if there’s any visible separation or layering over time in a liquid or gas sample.

2. Phase Separation Techniques

Mixtures can often be separated by physical means while substances cannot:
- Filtration: This technique separates a solid from a liquid by passing the mixture through a filter medium. The solid will remain on the filter, and the liquid will pass through.
- Distillation: This process uses differences in boiling points to separate mixtures with different components, particularly useful for liquid solutions.
- Chromatography: Ideal for separating mixtures where components have different affinities for a stationary phase and a mobile phase.
🔎 Note: Not all substances or mixtures can be effectively separated using these methods. Some complex mixtures might require advanced techniques like electrophoresis or gas chromatography.
3. Chemical Analysis

Chemical reactions can reveal whether a material is a substance or a mixture:
- When a substance undergoes a chemical change, it reacts as a single entity. The reaction will produce consistent products from identical conditions.
- Mixtures might show varying results when subjected to the same conditions, as different components react differently. This can be observed through chemical tests, such as:
- pH testing for reactive components in a mixture.
- Precipitation reactions to identify ionic compounds.
- Combustion analysis to determine the empirical formula of a substance or detect impurities in mixtures.
4. Physical Properties

Physical properties like melting and boiling points offer clues:
- Substances have fixed melting and boiling points, as all molecules within the sample are the same.
- Mixtures might not have a sharp melting point, instead showing a range over which melting or boiling occurs due to varying component proportions.
| Property | Substance | Mixture |
|---|---|---|
| Melting Point | Sharp | Broad Range |
| Boiling Point | Sharp | Broad Range |
| Solubility | Consistent | Can Vary |

✅ Note: Real-world substances can contain trace impurities, which might slightly affect their properties, making precise differentiation sometimes challenging.
5. Spectroscopic Techniques

Spectroscopy provides a detailed view of a material’s molecular composition:
- Infrared Spectroscopy (IR): Measures how well substances absorb light at different wavelengths, revealing functional groups in the sample.
- Mass Spectrometry: Identifies the mass of compounds or components within a sample, helping to differentiate substances from mixtures.
- Nuclear Magnetic Resonance (NMR): Provides information on molecular structure, useful for both complex mixtures and pure substances.
To differentiate substances from mixtures using spectroscopy, look for:
- Single, sharp peaks in IR or NMR spectra for substances.
- Multiple peaks or broad signals in mixtures, indicating multiple molecular species.

By employing these five methods, you can gain a clearer understanding of whether you're dealing with a pure substance or a mixture. Each technique has its strengths, and sometimes combining methods yields the most accurate results.
The differentiation of substances from mixtures has widespread implications. In pharmaceuticals, it ensures drug purity; in environmental science, it aids in the analysis of pollutants; and in forensic science, it helps in substance identification. Whether you're a student learning the basics of chemistry or an expert in the field, recognizing these differences can open up new avenues of exploration and application.
Why is it important to differentiate between substances and mixtures?

+
Understanding the difference is critical for quality control, purity in pharmaceuticals, safety in food production, environmental analysis, and research in chemistry and materials science.
Can a mixture become a substance?

+
Not directly, but through a process called chemical synthesis, different compounds in a mixture can react to form a new substance with uniform properties.
What is the significance of spectroscopy in mixture analysis?

+
Spectroscopy allows for non-destructive analysis, providing molecular fingerprints that can help identify components within complex mixtures, assess purity, and even quantify the presence of substances.
How can substances and mixtures behave similarly?

+
Both can exhibit similar physical properties like color, density, or even form solutions when dissolved in certain solvents. This similarity can sometimes confuse casual observers.
Are all substances chemically pure?

+
While substances have a uniform composition, real-world examples might contain trace impurities. Chemically pure means the substance is free from any foreign atoms or molecules, which is rare in practical scenarios.



