5 Key Facts: Subatomic Particles and Isotopes Explained

In the world of physics, chemistry, and atomic science, understanding the basic building blocks of matter is fundamental. Subatomic particles and isotopes are pivotal concepts that illuminate how elements behave, interact, and form the universe as we know it. This post delves into the intricacies of subatomic particles—the protons, neutrons, and electrons—and the fascinating phenomenon of isotopes to provide a comprehensive overview for anyone curious about the microcosmic dance of atoms.
Understanding Subatomic Particles
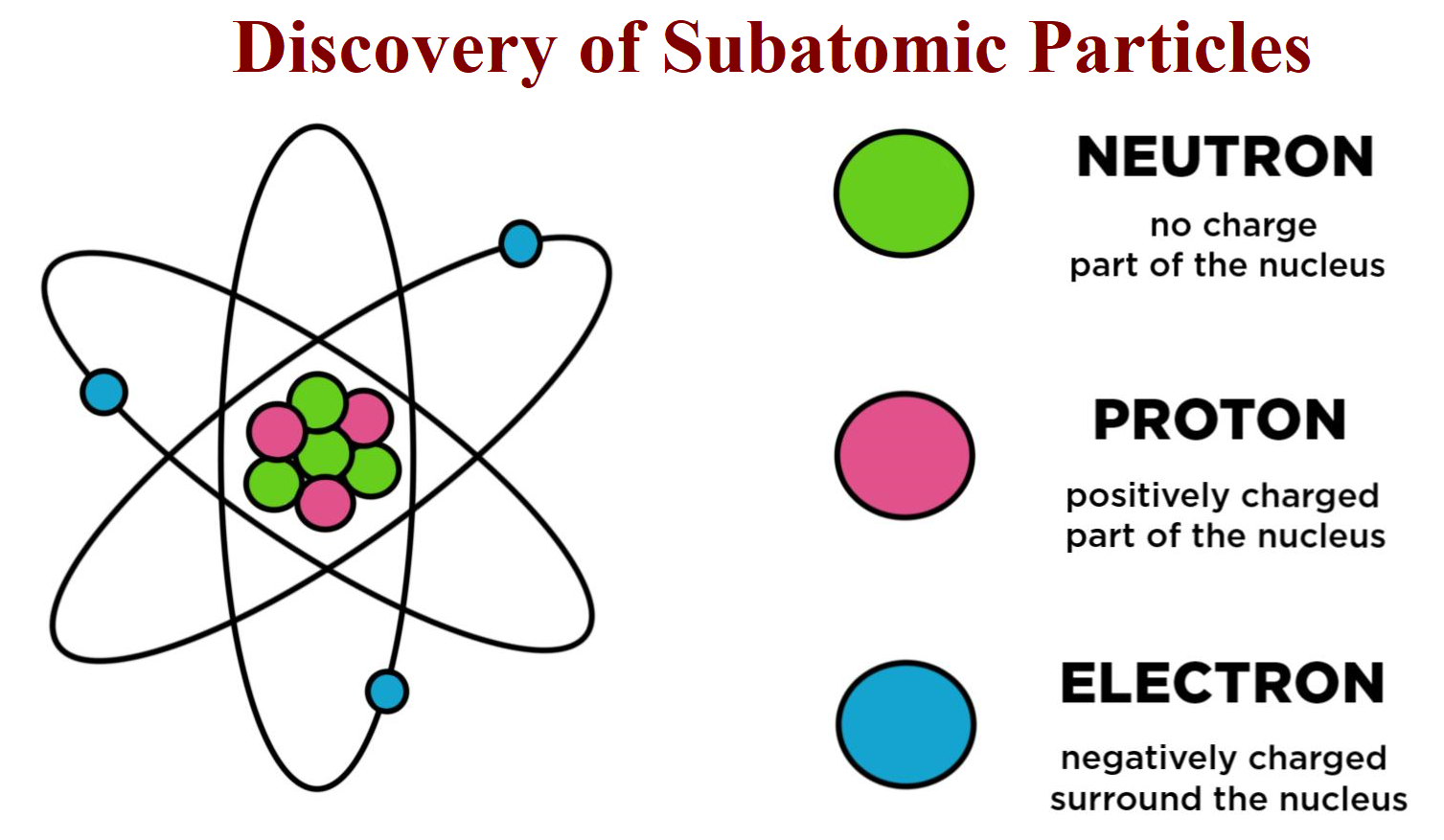

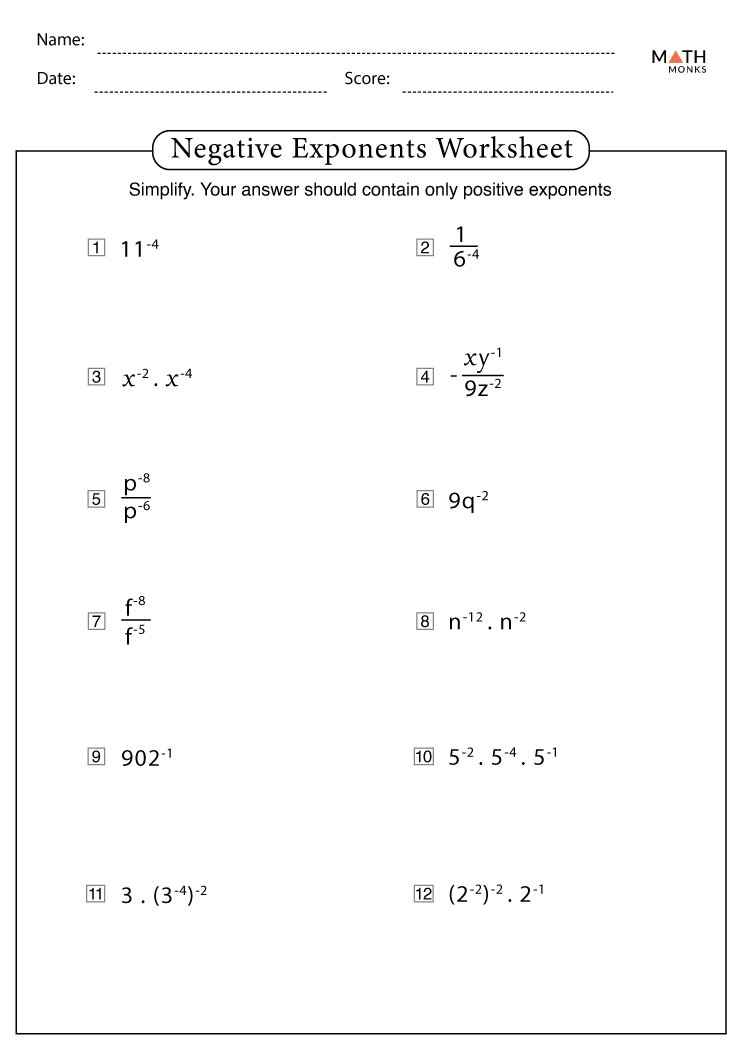
Subatomic particles are the smallest known particles of an atom, each playing a crucial role in its identity and characteristics:
- Protons: Positively charged particles located in the nucleus of an atom. They define the atomic number of an element.
- Neutrons: Neutral particles also in the nucleus, contributing to the mass number of an atom without affecting its charge.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells. They are involved in chemical reactions.
⚛️ Note: The term "subatomic" refers to particles smaller than an atom, which once thought to be indivisible, has been disproven by modern physics.
The Role of Protons

Protons are key to defining what an element is. Here’s what you should know:
- The number of protons in an atom’s nucleus determines its atomic number, which in turn specifies the element’s identity.
- Protons have a mass approximately 1836 times that of an electron.
- In neutral atoms, the number of protons equals the number of electrons.
Neutrons and Atomic Mass
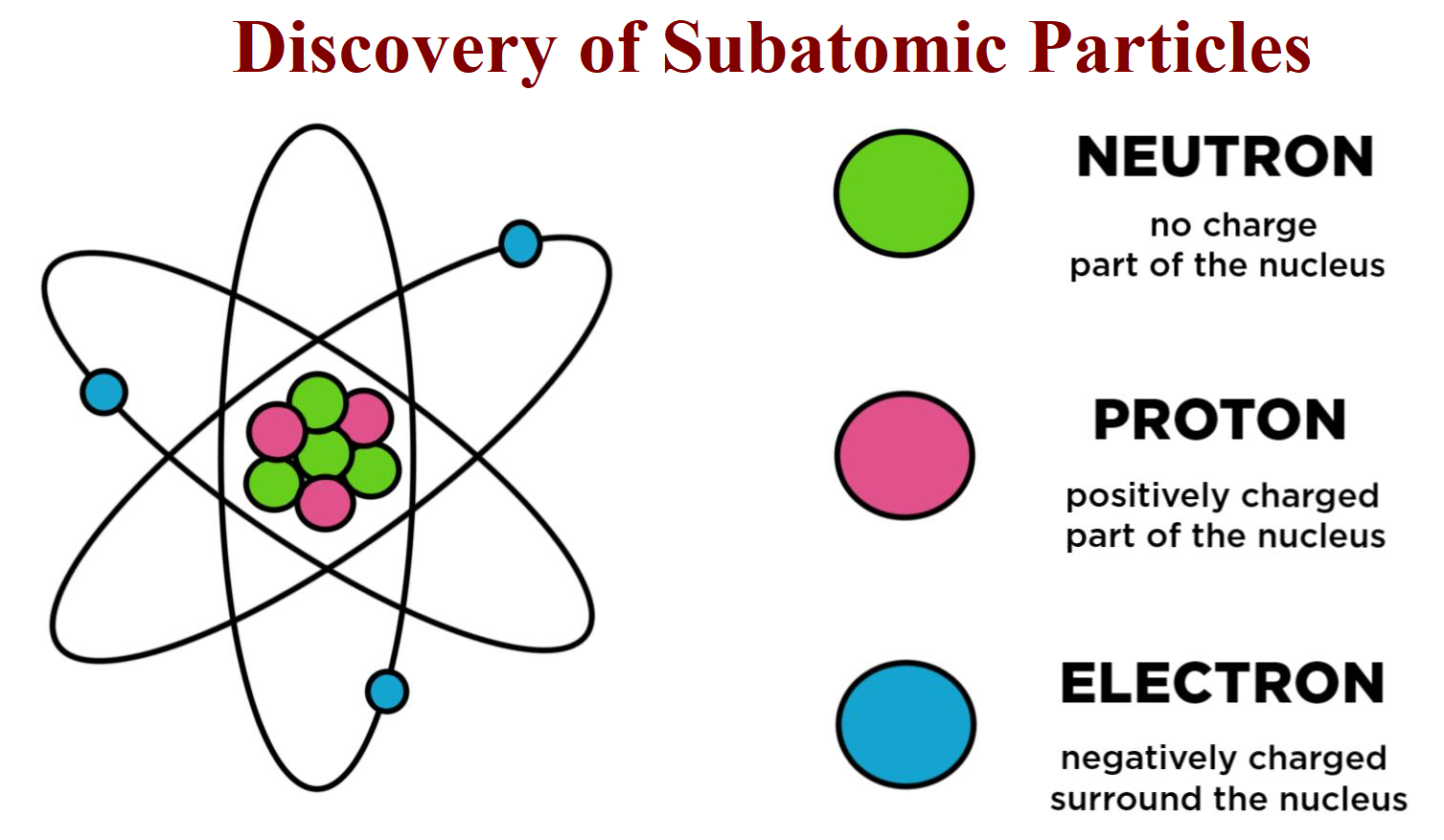
Neutrons play a subtle yet significant role:
- They contribute to the mass of an atom without altering its charge.
- Isotopes of the same element differ in the number of neutrons.
- They help stabilize the nucleus, influencing radioactivity.
Exploring Isotopes


Isotopes are variants of elements with the same number of protons but different numbers of neutrons. This variation in neutron count leads to:
- Varied mass number for each isotope of an element.
- Isotopes can be stable or radioactive, influencing the element’s behavior and applications.
| Element | Isotope | Protons | Neutrons | Mass Number | Common Use |
|---|---|---|---|---|---|
| Carbon | C-12 | 6 | 6 | 12 | Basis for atomic mass scale |
| Carbon | C-14 | 6 | 8 | 14 | Radioactive dating |
| Uranium | U-235 | 92 | 143 | 235 | Nuclear reactors, weapons |
| Uranium | U-238 | 92 | 146 | 238 | Nuclear waste |

🧑🔬 Note: The mass number is the sum of protons and neutrons in an atom’s nucleus.
The Electron’s Dance

While the nucleus is the atom’s core, electrons are what give it life in chemical interactions:
- They occupy energy levels around the nucleus in orbits or orbitals.
- Changes in electron configuration lead to chemical reactions.
- Electrons are key in bonding, forming compounds, and in electrical conductivity.
The Importance of Isotopes

Isotopes are not just interesting variations; they have practical applications:
- Medical Applications: I-131, a radioactive isotope of iodine, is used in the treatment of thyroid cancer.
- Agriculture: Certain isotopes help in studying plant growth and soil conditions.
- Archaeology: Carbon-14 dating allows for the age determination of organic material.
In essence, the study of subatomic particles and isotopes reveals the intricate details of matter's behavior and the fundamental particles that constitute our world. From defining what makes an element unique to understanding its practical applications, these concepts are cornerstones of scientific knowledge and progress.
What are subatomic particles?

+
Subatomic particles are particles that make up atoms. The primary subatomic particles are protons, neutrons, and electrons, each with distinct charges and roles within the atom.
How do isotopes differ from regular atoms?

+
Isotopes are atoms of the same element with different numbers of neutrons, leading to differences in mass number but not in chemical behavior since the atomic number (proton count) remains the same.
Why are isotopes important in nuclear technology?

+
Isotopes like Uranium-235 are critical for nuclear reactors and weapons due to their radioactive properties. Different isotopes have unique nuclear properties that make them suitable for specific applications.