Master Mole to Mole Stoichiometry with Our Worksheet Answers

Learning chemistry, particularly stoichiometry, can be both fascinating and challenging. If you're struggling with understanding mole to mole stoichiometry, you're not alone. However, with the right guidance, including detailed worksheet answers, you can master this critical aspect of chemical calculations. Here's a comprehensive guide to help you navigate through mole to mole stoichiometry with confidence.
Understanding Stoichiometry

Stoichiometry is at the heart of chemical reactions, detailing the quantitative relationships among substances as they react to form products. Before diving into specific worksheet problems, it’s crucial to understand some key concepts:
- Moles: The mole is a unit in chemistry that indicates the amount of a substance. One mole of any substance contains Avogadro’s number of particles (6.022 x 10^23).
- Molar Mass: The mass of one mole of a substance, typically given in grams per mole (g/mol).
- Balanced Chemical Equations: The chemical equation must be balanced to show the correct stoichiometric relationship between reactants and products.
📚 Note: Always ensure the equation is balanced before you begin stoichiometric calculations to avoid errors.
Mole to Mole Relationships in Chemical Equations
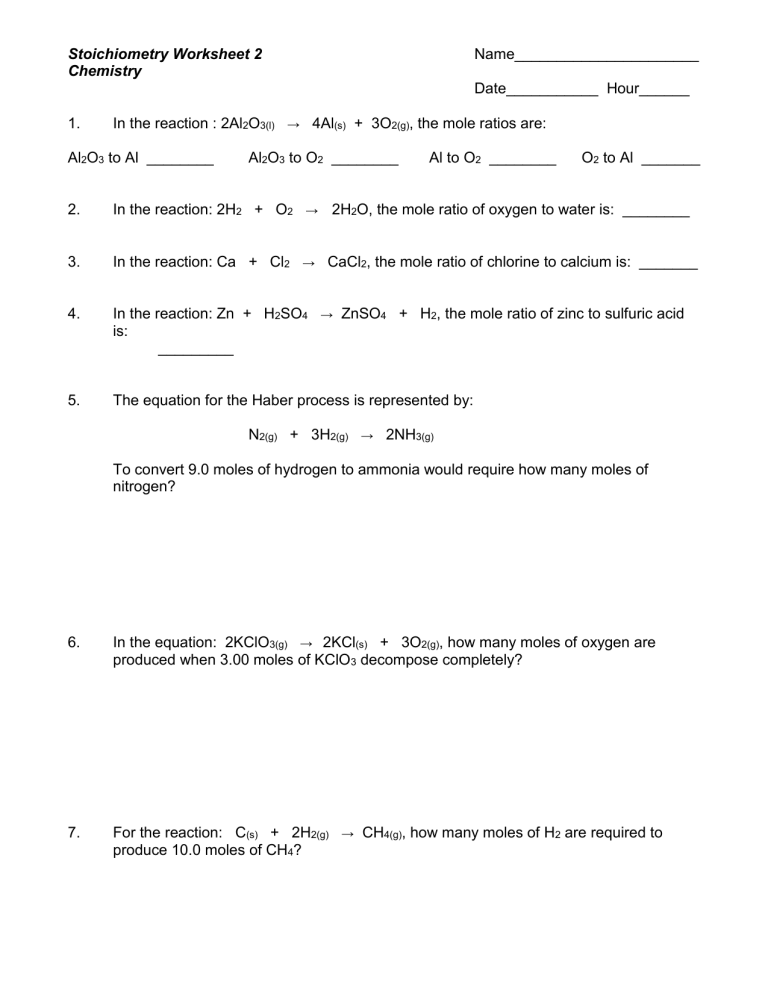
A balanced chemical equation provides the mole ratios between substances. Here’s how to interpret these relationships:
- If you know the amount of one substance, you can calculate how much of another substance will be produced or consumed by using the ratio of coefficients in the balanced equation.
- For example, in the reaction 2H2 + O2 → 2H2O, 2 moles of hydrogen gas react with 1 mole of oxygen gas to produce 2 moles of water.
How to Solve Mole to Mole Stoichiometry Problems

Here’s a step-by-step guide to solving stoichiometry problems:
- Write the Balanced Equation: Ensure the chemical equation you are working with is correctly balanced.
- Identify the Known and Unknown Moles: Determine what you know (in moles) and what you need to find out (also in moles).
- Find the Mole Ratio: Use the coefficients from the balanced equation to set up a ratio between the known substance and the unknown substance.
- Set up Proportions: Use the mole ratio to set up a proportion for calculating the unknown.
- Solve for the Unknown: Multiply and divide as per the set proportion to find the moles of the unknown substance.
Worksheet Example

Let’s apply these steps to an example problem:
Problem: If you have 3 moles of sodium bicarbonate (NaHCO3) and it reacts with hydrochloric acid according to the equation:
NaHCO3 + HCl → NaCl + H2O + CO2
How many moles of carbon dioxide (CO2) will be produced?
- The equation is balanced.
- We have 3 moles of NaHCO3 and need to find the moles of CO2.
- The mole ratio from the equation is 1:1 for NaHCO3 to CO2.
- Set up the proportion:
1 mol NaHCO3 / 1 mol CO2 = 3 mol NaHCO3 / x mol CO2 - Solving for x:
x = (1 * 3) / 1 = 3 mol CO2
Hence, 3 moles of CO2 will be produced from 3 moles of NaHCO3.
Advanced Stoichiometry Calculations

Here are some more complex scenarios:
- Multiple Products: If a reaction produces more than one product, you can still use the mole ratios from the equation to calculate each product’s yield.
- Limiting Reactant: When reactants are not provided in stoichiometric ratios, you need to identify which reactant will run out first, limiting the product yield.
Tips for Mastering Stoichiometry

- Practice with Various Equations: The more you practice with different types of equations, the better you’ll understand stoichiometry.
- Check Your Units: Ensure all calculations are consistent with units, particularly moles.
- Understand Limiting Reagent Problems: These often appear in exams and are key to mastering stoichiometry.
🔍 Note: If you’re dealing with fractions, use common denominators or simplify the math for easier calculations.
In wrapping up this discussion, mastering mole to mole stoichiometry involves a clear understanding of balanced chemical equations, mole ratios, and the step-by-step process of setting up and solving stoichiometric problems. By following the guidelines provided and working through example problems, you can develop a solid foundation in this essential aspect of chemistry. Whether you're solving for the yield of a reaction or understanding the mass relationships between reactants and products, stoichiometry is the tool that links theoretical chemistry with practical applications. Keep practicing, apply the concepts learned here, and you'll soon find stoichiometry not just understandable, but manageable and even enjoyable.
What is stoichiometry?

+
Stoichiometry is the part of chemistry concerned with the calculation of the relative quantities of reactants and products in chemical reactions. It deals with the quantitative relationships among substances in these reactions.
Why is stoichiometry important?

+
It’s essential for predicting the yield of a reaction, understanding how chemicals react, designing chemical reactions, and is critical in fields like pharmaceuticals, environmental science, and industrial processes.
How do you find the limiting reactant in a reaction?

+
To find the limiting reactant, you calculate how much product each reactant would produce if it were completely consumed. The reactant that produces the least amount of product is the limiting reactant.



