Solubility Worksheet Answers: Simplify Your Chemistry Studies
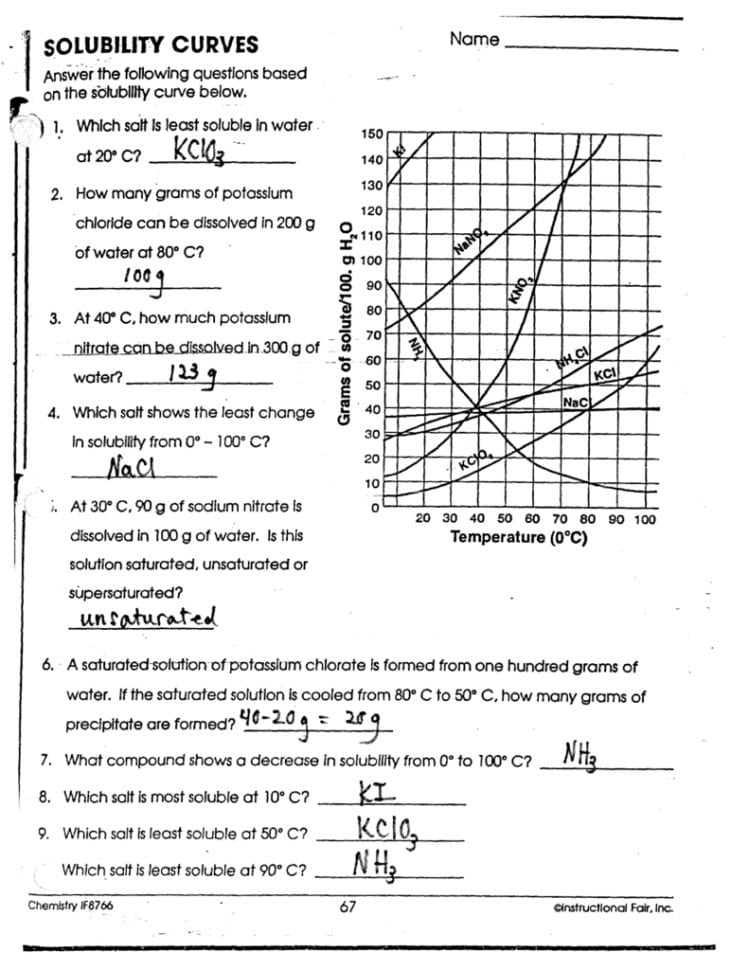
Understanding Solubility: A Comprehensive Guide to Solubility Rules and Practice Problems
Solubility is a fundamental concept in chemistry, often encountered when discussing solutions, reactions, and material properties. Whether you're a student grappling with chemistry homework or someone with a keen interest in understanding how substances interact in various environments, this blog post will delve into the intricacies of solubility rules and provide a comprehensive set of solubility worksheet answers to aid your learning. This guide will help you understand, apply, and practice solubility rules to simplify your chemistry studies.
What is Solubility?

Solubility refers to the maximum amount of solute that can dissolve in a solvent at a given temperature and pressure, resulting in a homogeneous solution. Here are some key points to remember:
- Homogeneous Solutions: The solute and solvent are uniformly mixed, and the solution appears as one phase.
- Saturation: A solution is saturated when it contains the maximum amount of solute that can dissolve at the given conditions.
- Units of Solubility: It is typically measured in grams per liter (g/L) or moles per liter (mol/L).
Factors Influencing Solubility

Solubility isn't static; it's influenced by:
- Temperature: Generally, increasing the temperature increases the solubility of solids in liquids.
- Pressure: For gases dissolved in liquids, an increase in pressure increases solubility (Henry's Law).
- Chemical Composition: The nature of solute and solvent affects solubility due to forces like ionic interactions and polarity.

Essential Solubility Rules

To simplify the understanding of which compounds dissolve and which do not, chemists have developed solubility rules. Here's a summary:
- All Group 1 salts (alkali metals) are soluble.
- Salts containing nitrates (NO₃⁻), acetates (CH₃COO⁻), and ammonium ions (NH₄⁺) are soluble.
- Chlorides (Cl⁻), Bromides (Br⁻), and Iodides (I⁻) are soluble, except those of Ag⁺, Pb²⁺, and Hg₂²⁺.
- Salts containing sulfates (SO₄²⁻) are soluble except those of Ca²⁺, Sr²⁺, Ba²⁺, Pb²⁺, and Hg₂²⁺.
- Most carbonates (CO₃²⁻) and phosphates (PO₄³⁻) are insoluble, except those of alkali metals and NH₄⁺.
- Hydroxides (OH⁻) are insoluble except for those of Group 1 metals and NH₄⁺.
Mastering Solubility Rules

To internalize these rules:
- Visualize solubility flowcharts or tables.
- Use mnemonic devices like songs or acronyms to remember exceptions.
- Practice with solubility problems to cement your understanding.
🔍 Note: Remember that the solubility of ionic compounds can sometimes be influenced by pH, presence of common ions, or complex formation.
Solubility Worksheet Answers: Practice with Explanations

Here are some solubility worksheet answers to help you practice and understand the application of solubility rules:
Problem 1: Determine the solubility of each compound in water:

- KNO₃: Soluble due to nitrate rule.
- AgCl: Insoluble due to exceptions to chloride rule.
- Na₂CO₃: Soluble as a Group 1 metal salt.
| Compound | Is it Soluble? |
|---|---|
| KNO₃ | Yes |
| AgCl | No |
| Na₂CO₃ | Yes |

Problem 2: Explain why AgCl is insoluble in water:

Silver chloride (AgCl) is not soluble because of the exception to the chloride solubility rule. While many chlorides are soluble, silver chloride, lead chloride, and mercury(II) chloride are notable exceptions.
🔍 Note: Despite its insolubility, silver chloride forms very slightly soluble complexes with ammonia or cyanide ions.
Problem 3: What happens when you add Na₂SO₄ to a solution of BaCl₂?

Barium sulfate (BaSO₄) will precipitate because:
- Both Ba²⁺ and SO₄²⁻ ions are present in the solution.
- Barium sulfate is one of the exceptions to the sulfate solubility rule.
🔍 Note: A double displacement reaction will occur, leading to the formation of BaSO₄ which will settle out due to its low solubility in water.
This process of simplifying solubility rules through structured problem-solving not only aids in retaining the information but also fosters a deeper understanding of chemical reactions and solution properties.
In wrapping up, solubility is more than just a chemical phenomenon; it’s a cornerstone of how substances interact. By mastering solubility rules and practicing solubility worksheet answers, you’ve taken a significant step in simplifying your chemistry studies. The ability to predict whether substances will dissolve, react, or precipitate opens up new avenues for understanding complex chemical systems. Whether for academic success or practical applications in industries like pharmaceuticals or environmental science, your newfound solubility knowledge is invaluable. Keep exploring and applying these principles, and you’ll find chemistry to be not just manageable but fascinating.
Why are Group 1 metal salts always soluble in water?

+
Group 1 metal salts are soluble because the alkali metal ions have a strong hydration energy that overcomes their lattice energy, making it easy for them to dissolve in water.
What is the common ion effect?

+
The common ion effect is the lowering of the solubility of an ionic compound when a salt that contains an ion already present in the compound is added to the solution. This reduces the solubility because the equilibrium shifts to the less soluble side.
How do I predict if an ionic compound will form a precipitate?

+
To predict if an ionic compound will form a precipitate, use solubility rules. If the combination of ions results in a compound that is insoluble according to these rules, a precipitate will form.