Mastering Protons, Neutrons, Electrons: Practice Worksheet Guide

In the vast universe of chemistry, understanding the basic building blocks of matter is crucial for budding scientists and established researchers alike. At the heart of this knowledge are protons, neutrons, and electrons, the fundamental particles that make up atoms. If you're here, it's probably because you're keen on delving into these mysteries, possibly for an upcoming test or to solidify your comprehension of atomic structures. In this detailed guide, we'll explore the concepts of protons, neutrons, and electrons, how they interact, and provide you with a thorough practice worksheet designed to elevate your understanding. Let's start this journey through the atom!
The Atomic Landscape

Before we plunge into worksheets and practice problems, let’s lay the groundwork with a quick overview:
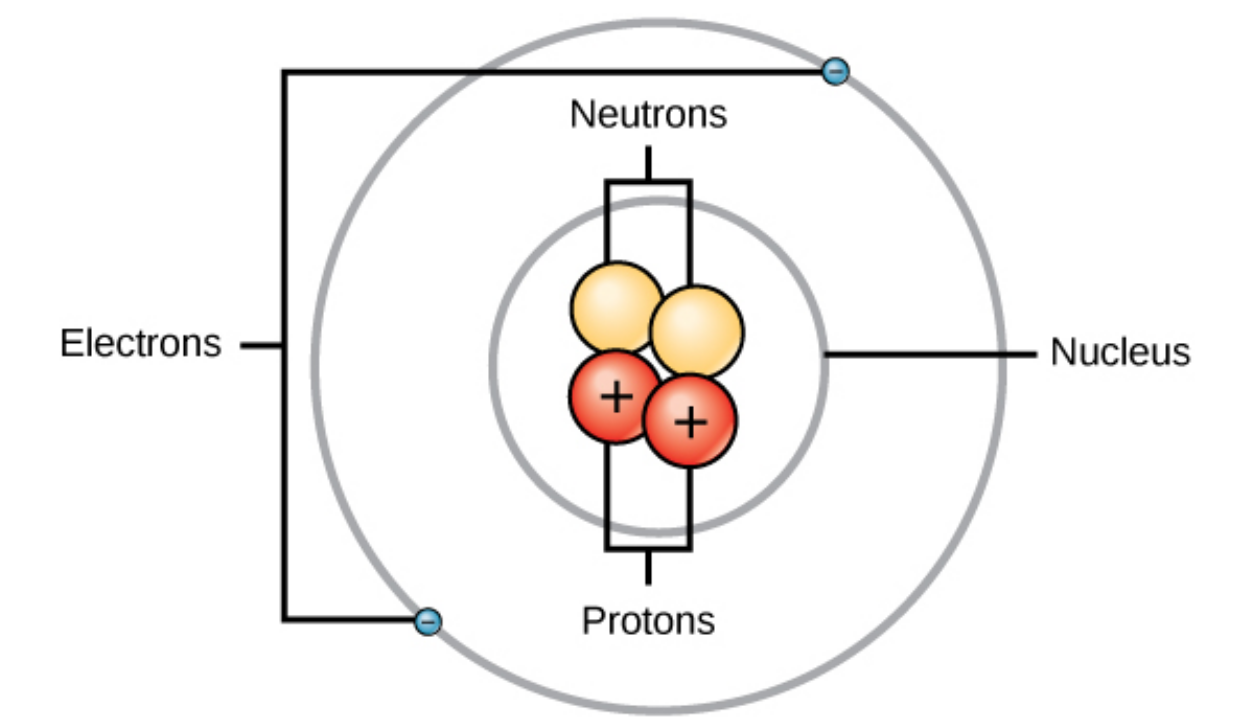
- Protons - These are positively charged particles found in the nucleus of an atom. They determine an atom’s atomic number.
- Neutrons - These are neutrally charged particles also residing in the nucleus, affecting the atom’s mass but not its chemical identity.
- Electrons - These negatively charged particles orbit the nucleus, involved in chemical reactions and bonding.
The interplay between these particles dictates the behavior and properties of elements on the periodic table. With this backdrop, let’s dive into our practice worksheet.

Practice Worksheet: Understanding Protons, Neutrons, and Electrons

This worksheet is designed to help you grasp the concepts of atomic particles through problem-solving. Here’s how you can approach each type of problem:
Question 1: Identifying Atomic Particles

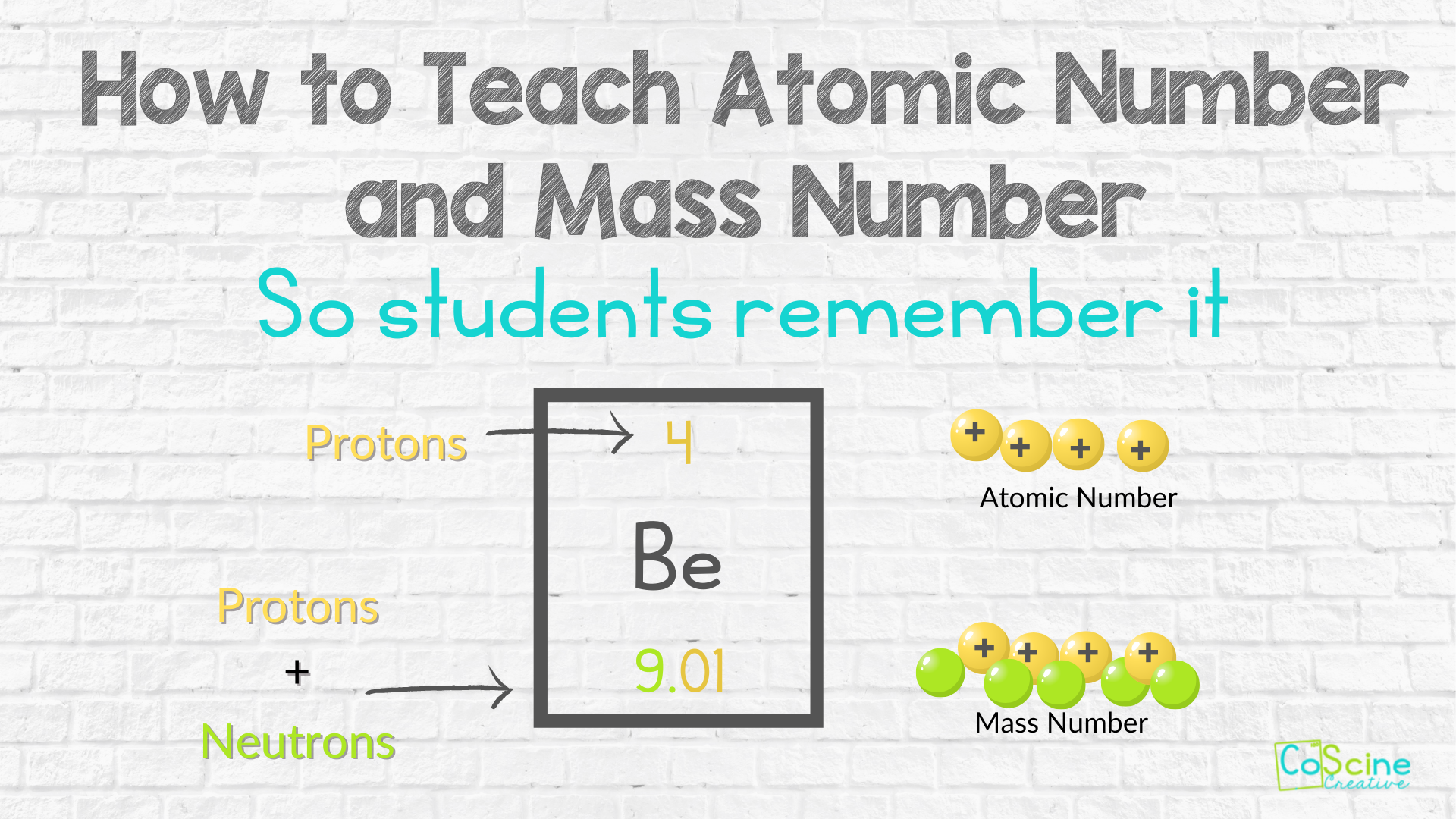
Problem: Given an element with atomic number 15 (Phosphorus), what is the number of protons, electrons, and neutrons in its neutral atom?
- Step 1: Identify Protons - The atomic number gives you the number of protons, which is 15 for phosphorus.
- Step 2: Determine Electrons - In a neutral atom, the number of protons equals the number of electrons, so you also have 15 electrons.
- Step 3: Calculate Neutrons - For this, you’ll need the mass number, which isn’t given. However, assuming a common isotope (Phosphorus-31), the mass number is 31, giving you 31 - 15 = 16 neutrons.
📝 Note: For a more accurate neutron count, you might need to refer to the periodic table for the atomic mass, which is an average of all isotopes' masses.
Question 2: Isotopes

Problem: How many neutrons are in a potassium isotope with a mass number of 41?
- Step 1: Look Up Protons - Potassium has an atomic number of 19.
- Step 2: Calculate Neutrons - Subtract the atomic number from the mass number: 41 - 19 = 22 neutrons.
Question 3: Electron Configuration
Problem: Write the electron configuration for the neutral atom of sodium (Na).
- Step 1: Determine Electron Number - Sodium has 11 electrons.
- Step 2: Arrange in Orbitals - Fill the electron shells: 1s² 2s² 2p⁶ 3s¹.
📝 Note: Remember that the electron configuration of an element in its neutral state gives the number of electrons in its ground state configuration.
Table: Atomic Mass Contributions

| Particle | Charge | Mass Contribution (amu) |
|---|---|---|
| Proton | +1 | 1 |
| Neutron | 0 | 1 |
| Electron | -1 | ~0.0005 |
The table above underscores that while protons and neutrons have significant mass contributions, electrons are so light, their mass can be nearly negligible when calculating atomic mass.
Summing it All Up

Your journey through protons, neutrons, and electrons not only equips you with the necessary knowledge to tackle chemistry problems but also enhances your understanding of how matter behaves at its most fundamental level. By engaging with this worksheet, you’ve practiced identifying particles, understanding isotopes, and learning about electron configurations, which are essential in describing atomic interactions. These practices are the building blocks for a deeper comprehension of chemistry, from bonding to periodic trends. Keep practicing, and soon, you’ll navigate these atomic landscapes with confidence!
What role do isotopes play in chemistry?

+
Isotopes are atoms of the same element with different numbers of neutrons. They play crucial roles in various fields like medicine (radiotherapy), archaeology (carbon dating), and environmental science (tracer studies). The difference in neutron count affects the atomic mass but not chemical reactivity, making isotopes useful for specific applications where additional neutrons don’t alter chemical behavior.
How does electron configuration influence atomic behavior?

+
Electron configuration defines how atoms form bonds with one another, determining an element’s chemical properties. It dictates the valence electrons available for bonding, the reactivity of an element, and how it might gain, lose, or share electrons. Understanding this configuration helps predict how elements will interact with others in chemical reactions.
Why are protons important in determining an element’s identity?

+
The number of protons, or the atomic number, defines an element’s identity because it determines its place in the periodic table. This number is unique to each element, setting it apart from all others chemically. Any change in proton count results in a different element entirely.



