5 Key Tips for Mastering Protons, Neutrons, and Electrons Practice
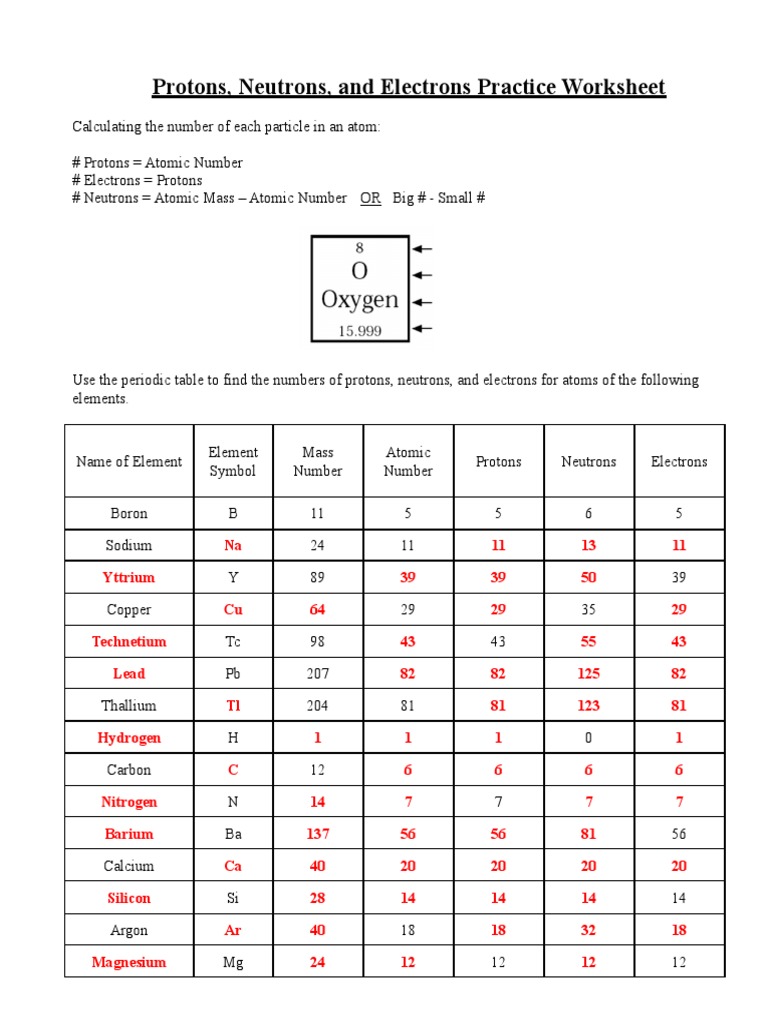
⚠️ Note: This post focuses on general study techniques for mastering chemistry, particularly the interaction of protons, neutrons, and electrons. It does not contain specific educational content on the topic.
Embarking on a journey to master the intricate world of protons, neutrons, and electrons in chemistry is not just about memorizing facts; it's about understanding the foundational concepts of matter and its behavior. Here, we’ll explore five key tips that will enhance your study routine and understanding of these subatomic particles, ensuring you stand out in your science education. Let’s dive in.
1. Visual Learning Through Models and Diagrams

- Use Physical Models: Invest in model kits or build simple models from scratch to visualize atomic structure. Manipulating physical objects can deepen your understanding of how protons, neutrons, and electrons work together.
- Diagrams and Illustrations: Complement your reading with diagrams. Sketching out the Bohr model or electron cloud model for different atoms can help consolidate your knowledge.
- Interactive Simulations: Online tools allow you to simulate electron configurations, atomic behavior, and interactions, providing a dynamic learning experience.
🌟 Note: Learning by doing enhances retention and understanding.
2. Understanding Through Real-World Applications

- Nuclear Reactions: Explore how understanding subatomic particles aids in nuclear reactions, from everyday uses like smoke detectors to complex processes in nuclear power plants.
- Radioactivity: Delve into how radioactive decay relates to subatomic particles, helping you grasp concepts like half-life.
- Chemical Bonding: Understand how electrons behave in bonding to form molecules, which underpins most of chemistry.
3. Employing Mnemonics and Memory Techniques

- Mnemonics: Use mnemonics for atomic numbers or electron configurations. For example, to remember the first 10 elements, you could use “Hydro, He-lium, Li-thium, Be-ryllium, Bo-ron, Carbon, Nitrogen, Oxygen, Flu-orine, Neon.”
- Acronyms: Develop acronyms to remember series of information. “PEMDAS” helps with order of operations; adapt this method to atomic structure.
- Storytelling: Create stories where protons, neutrons, and electrons are characters in a narrative, making the learning process enjoyable.
4. Active Recall and Spaced Repetition

Active recall is a powerful technique where you test yourself frequently on the material you’ve learned. Here’s how to integrate this into your study routine:
- Flashcards: Use them to test your knowledge of atomic weights, ion formations, or any other aspect of subatomic particles.
- Spaced Repetition: Instead of cramming, spread your study sessions over time to reinforce memory. Apps like Anki or physical review systems work wonders for this.
- Self-Quiz: Regularly take self-quizzes to gauge your understanding. This process not only reinforces learning but also highlights areas for further study.
👌 Note: Regular testing can significantly improve retention.
5. Peer Discussion and Study Groups

Engaging with peers can provide multiple perspectives and enhance learning:
- Discussion: Talk through concepts, question each other, and explain ideas in your own words. Teaching others can reveal gaps in your knowledge.
- Study Groups: Collaborate on assignments, share study materials, and test each other. Social interaction can make learning enjoyable and less stressful.
- Debate and Critique: Encourage peer critique on your understanding of nuclear models or chemical bonding, refining your knowledge.
Mastering the study of protons, neutrons, and electrons is about more than just the elements of a periodic table; it's about understanding the building blocks of matter. Through visualization, real-world connections, memory techniques, active learning, and collaborative study, you can transform your approach to chemistry from routine to revelatory. Remember, every new concept or model you learn is a step closer to unraveling the mysteries of the universe. Keep exploring, keep questioning, and above all, enjoy the learning journey.
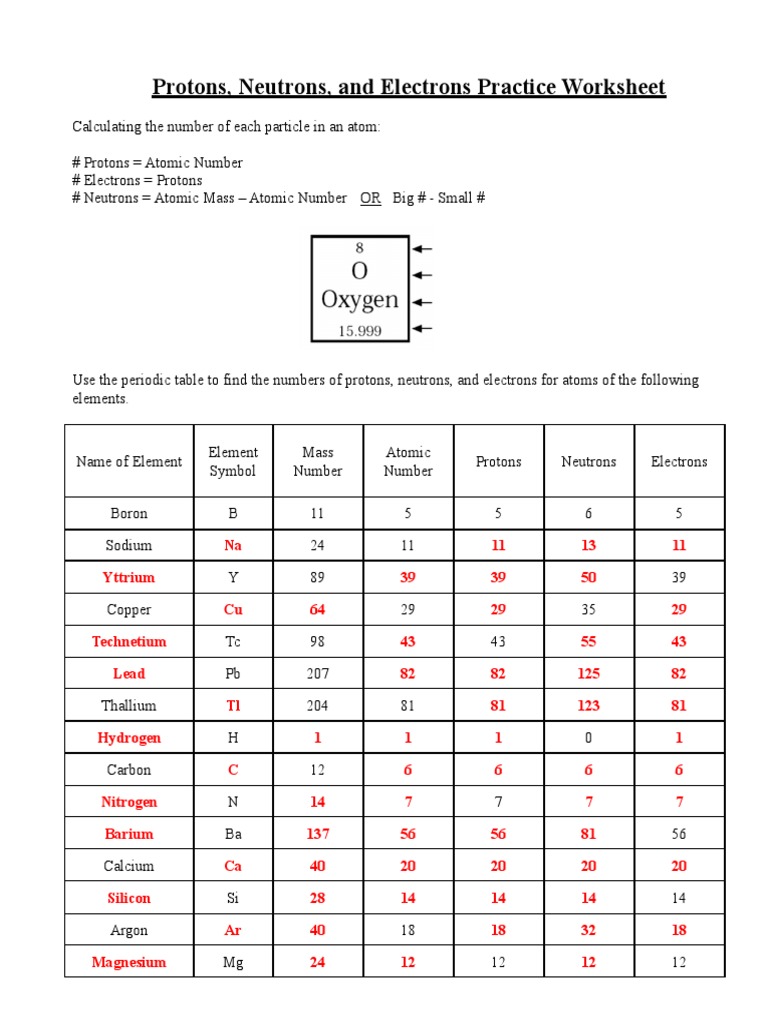
Why is understanding subatomic particles important?
+
Understanding protons, neutrons, and electrons is crucial because these particles determine the chemical properties, atomic mass, and the behavior of atoms. This knowledge forms the foundation for all chemical processes and reactions, which are integral to understanding fields from basic chemistry to advanced physics and beyond.
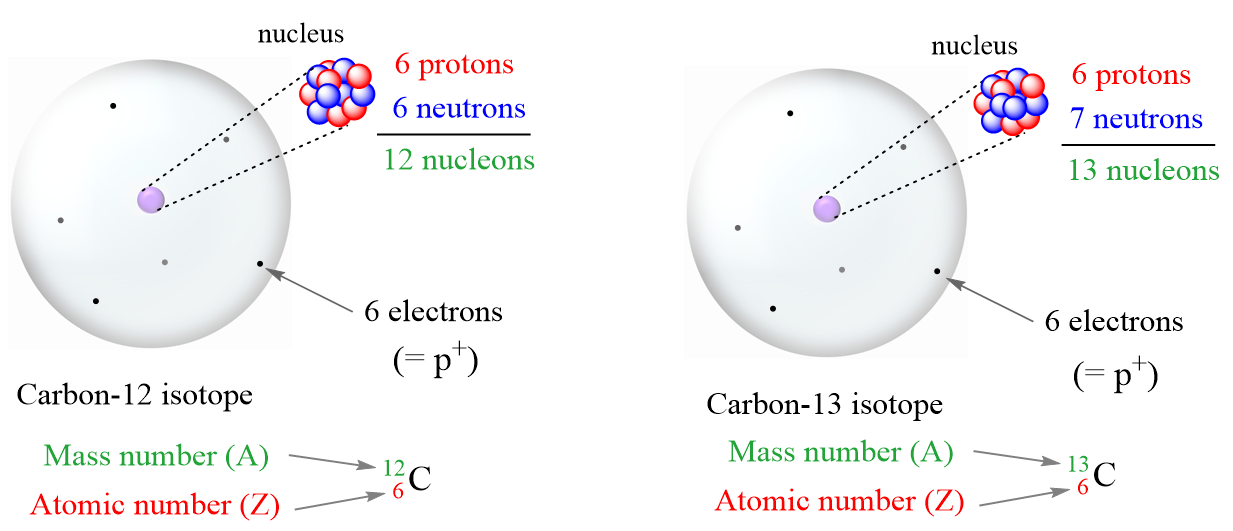
What are the best ways to study chemistry at the subatomic level?
+
Using a combination of visual learning, real-world applications, mnemonics, active recall, and peer discussions ensures comprehensive learning. Visual aids like diagrams and models can help you visualize atomic structures, while real-world applications make the concepts relatable. Memory techniques like mnemonics aid in memorization, active recall reinforces what you’ve learned, and peer discussions foster deeper understanding through diverse perspectives.
How can active recall benefit me in chemistry?

+
Active recall boosts memory retention by requiring you to retrieve information from your memory actively, which strengthens neural pathways. This method, paired with spaced repetition, can significantly improve long-term retention, making it easier to recall facts and concepts for exams or when solving chemistry problems.