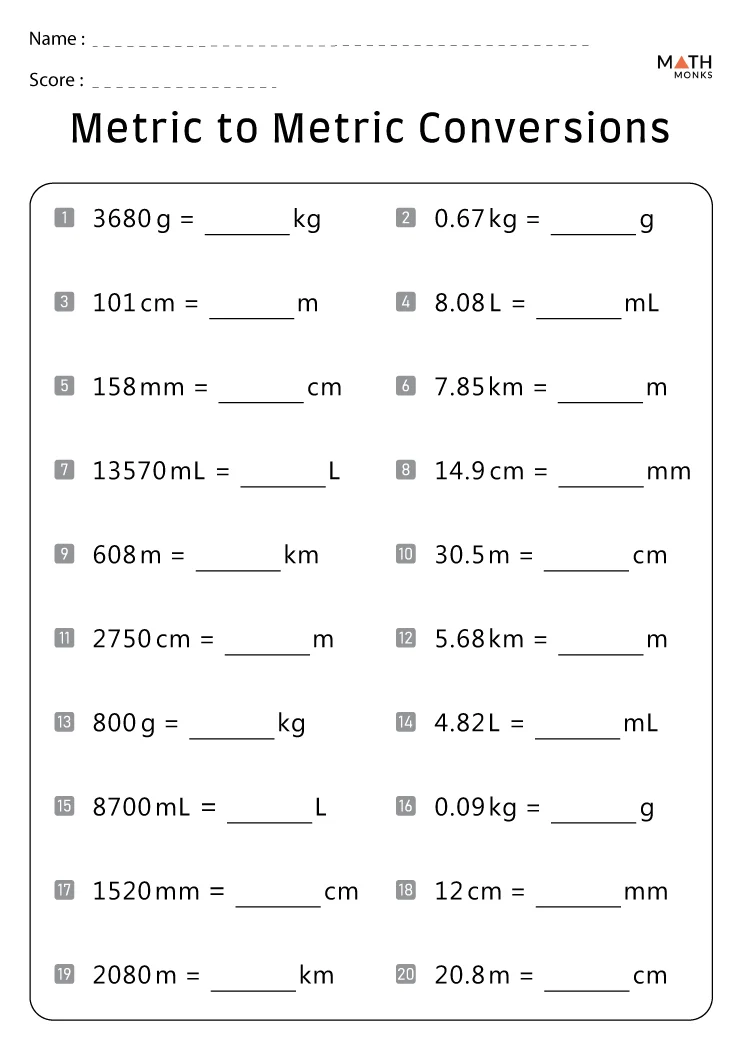
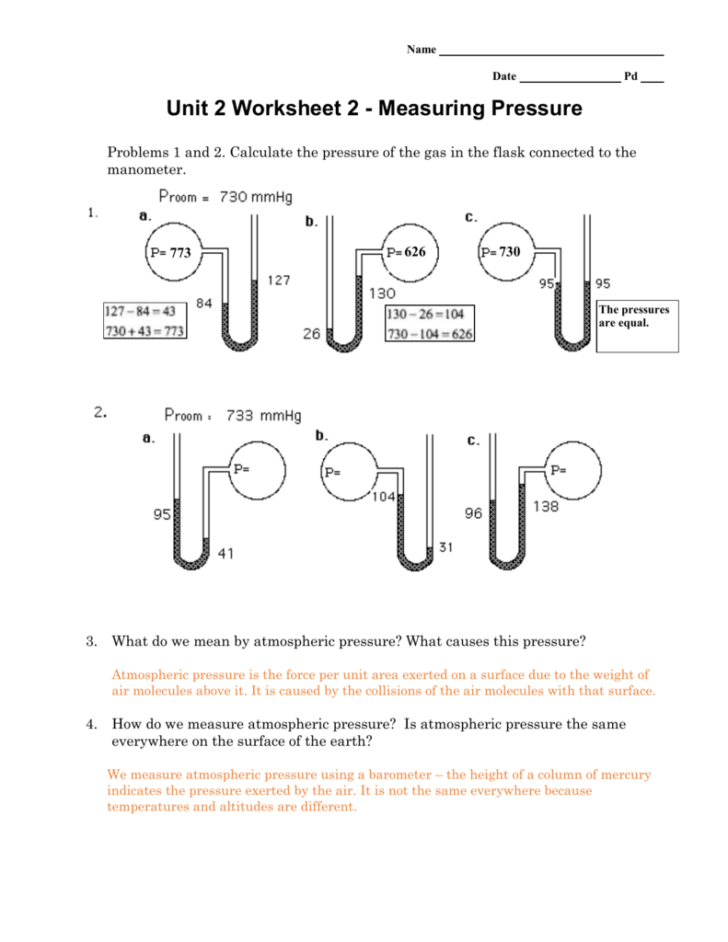
5 Easy Pressure Conversion Tips: Worksheet Answer Key
Whether you're a student learning about scientific measurements, an engineer dealing with complex systems, or simply someone intrigued by physics, understanding how to convert pressure from one unit to another is crucial. The nuances of pressure conversion can be initially challenging, but with these five easy tips, you'll unlock the secrets of unit conversion and breeze through your pressure-related tasks with ease.
Understanding Pressure Basics

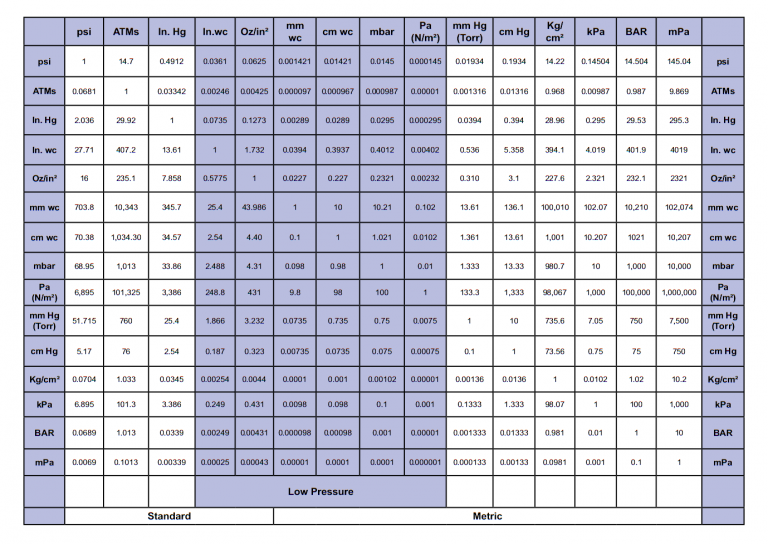
Pressure, the amount of force applied per unit area, is measured in various units worldwide. Familiarity with these units is the first step in mastering pressure conversion:
- Pascal (Pa): The SI unit for pressure, representing one newton per square meter (N/m²).
- Bar: Often used in meteorology and engineering, where 1 bar equals 100,000 Pa.
- Atmosphere (atm): Represents the average pressure at sea level, with 1 atm equivalent to approximately 101,325 Pa.
- Pounds per Square Inch (psi): Commonly used in the United States, where 1 psi is around 6,894.76 Pa.
- Millimeters of Mercury (mmHg): A pressure measurement often used in medicine, equaling about 133.322 Pa.
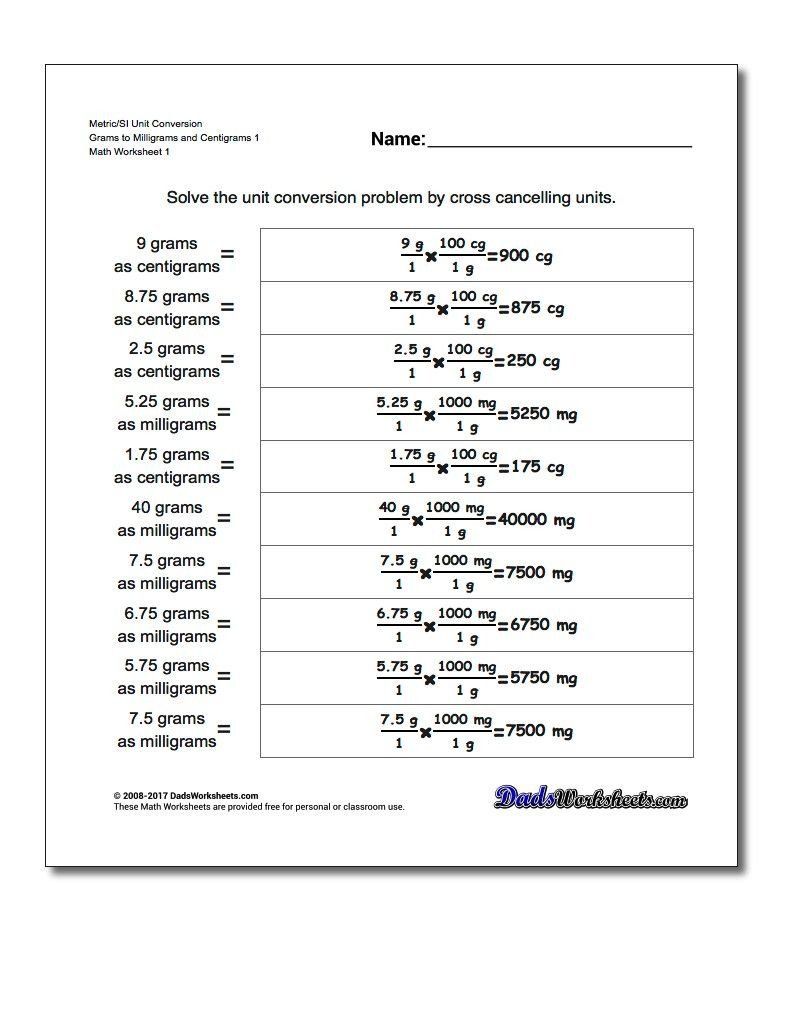
Tip 1: Memorize Key Conversion Factors

Memorizing some basic conversion factors is fundamental. Here are a few to remember:
- 1 atm = 101,325 Pa
- 1 bar = 100,000 Pa
- 1 psi = 6,894.76 Pa
- 1 mmHg = 133.322 Pa
Having these factors at your fingertips allows for quicker mental conversions.
Tip 2: Convert from the SI Unit

Converting pressure units can be easier if you first convert the value to Pascals, then convert from Pascals to your desired unit. Here's a simple example:
| Unit to convert | Conversion Factor to Pa |
|---|---|
| atm | 1 atm = 101,325 Pa |
| bar | 1 bar = 100,000 Pa |
| psi | 1 psi = 6,894.76 Pa |
| mmHg | 1 mmHg = 133.322 Pa |
Tip 3: Dimensional Analysis
Using dimensional analysis, or the factor-label method, is an effective strategy. By canceling out units, you can convert from one to another:
- Convert 2 atm to Pa:
2 atm * (101,325 Pa / 1 atm) = 202,650 Pa
This approach keeps your conversions structured and less error-prone.
Tip 4: Use Conversion Tools and Apps
In today's digital age, there's no shortage of tools to help you convert units. These include:
- Online conversion calculators
- Mobile apps like "Unit Converter" or "Convert Units"
- Spreadsheet software with built-in conversion formulas
These tools offer instant and accurate conversions, reducing the chance of errors when you're under time pressure or dealing with complex numbers.
Tip 5: Practice with Real-World Examples

Practice makes perfect. Here are some real-world examples to help you hone your skills:
- Convert the atmospheric pressure on a day when it's 1000 mb (millibars) to atm:
1000 mb * (1 bar / 1000 mb) * (1 atm / 1.01325 bar) ≈ 0.987 atm- Convert the pressure in a car tire from 30 psi to kPa:
30 psi * (6894.76 Pa / 1 psi) * (1 kPa / 1000 Pa) = 206.843 kPa- Calculate how many mmHg a pressure of 1013.25 hPa (hectopascal) would be:
1013.25 hPa * (100 Pa / 1 hPa) * (1 mmHg / 133.322 Pa) ≈ 760 mmHg
📝 Note: Always double-check your conversions, especially if the numbers seem significantly off.
Converting pressure units can seem daunting at first, but by understanding the basics, memorizing key conversion factors, using the SI unit as a stepping stone, applying dimensional analysis, leveraging conversion tools, and practicing with real-life examples, you'll find the process becoming second nature. Whether you're preparing for exams, solving engineering problems, or just exploring the world of physics, these tips will ensure that your pressure conversions are not only accurate but also efficient. With practice and the right approach, you'll master the art of pressure conversion and gain a deeper appreciation for the various units we use to measure the forces that shape our world.
Why do we have so many pressure units?
+
Pressure units have developed historically in various scientific, engineering, and practical contexts. Different professions and countries often developed their own standards, leading to a diverse range of units.
Can you convert pressure units without using Pascals?
+
Yes, while Pascals serve as the SI unit for pressure, you can directly convert from one non-SI unit to another using conversion factors, although using Pascals often simplifies the process.
What’s the significance of the atmosphere (atm) in pressure measurements?

+
The atmosphere (atm) represents the average air pressure at sea level, providing a baseline reference for atmospheric conditions. It’s commonly used in meteorology and physical sciences.
How do I handle non-standard pressure units?

+
Non-standard units can be handled by converting them first to a standard unit like Pascals or converting directly using known conversion factors between the units.
Are there any shortcuts for quick pressure conversions in everyday life?

+
In everyday situations, having a basic understanding of common units like atm, psi, and mmHg, and their approximate conversions can be a useful shortcut. However, for precision, always use reliable conversion tools or methods.



