5 Essential Tips for Ionic Charges Prediction

Predicting ionic charges in chemical compounds can be a challenging task for students and professionals alike in the field of chemistry. Understanding the rules and patterns that govern the behavior of atoms in ionic compounds not only helps in solving problems related to chemical reactions but also in predicting the properties of new compounds. Here are five essential tips to master the art of ionic charges prediction.
Understand the Periodic Table’s Group Number


The first step in predicting ionic charges involves a deep understanding of the periodic table. The group number of an element provides a clue:
- Group 1A (Alkali Metals): These elements generally form ions with a +1 charge.
- Group 2A (Alkaline Earth Metals): They tend to form ions with a +2 charge.
- Group 3A (Boron Group): Often form ions with a +3 charge, although exceptions exist.
- Group 4A (Carbon Group): Carbon and Silicon can form various charges depending on the bonding scenario.
- Group 5A (Nitrogen Group): Elements like Nitrogen can form both -3 and -2 ions.
- Group 6A (Oxygen Group): Typically form -2 ions.
- Group 7A (Halogens): These elements predominantly form ions with a -1 charge.
- Group 8A (Noble Gases): These are generally inert but can form positive ions in specific conditions.
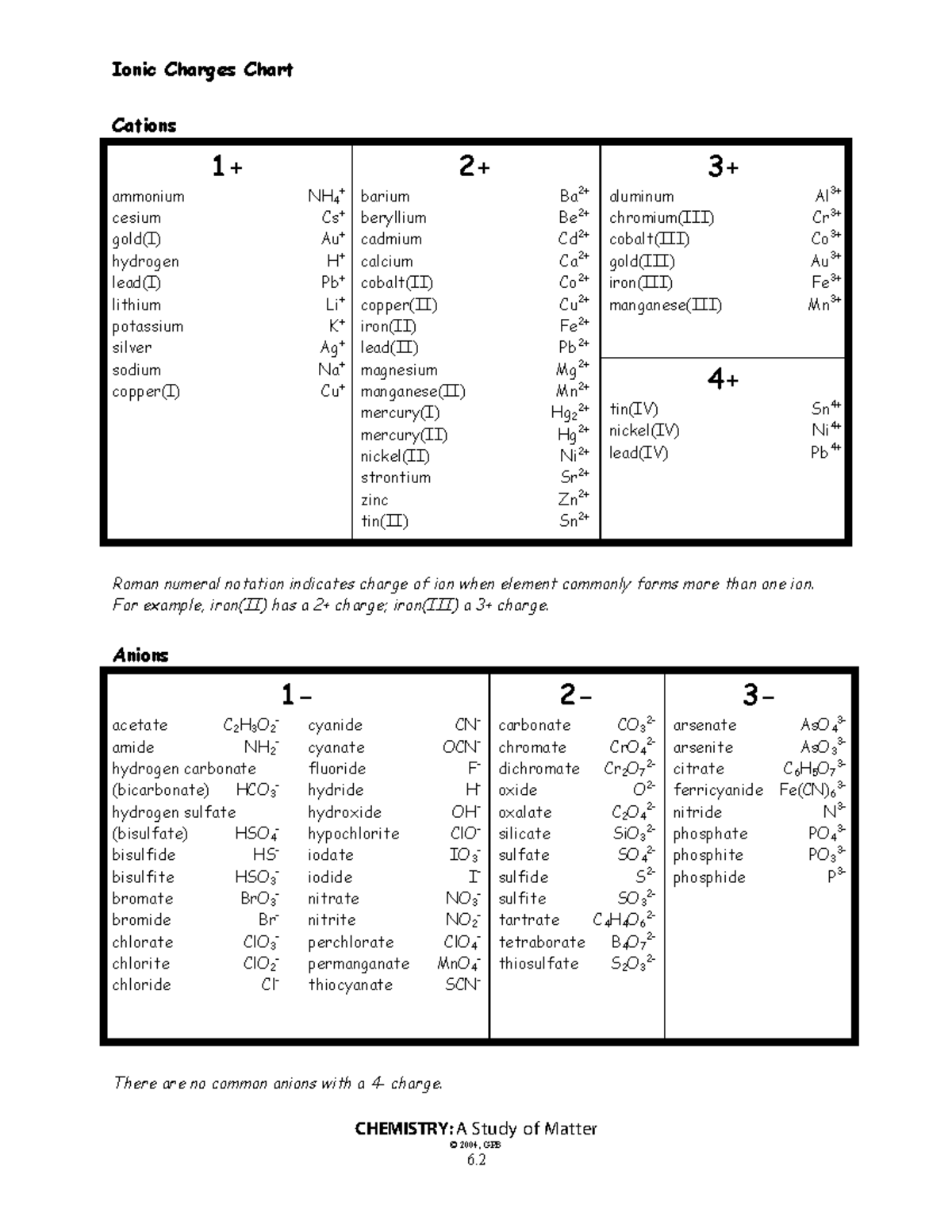
Recognize Common Polyatomic Ions

Polyatomic ions are groups of atoms covalently bonded together that have an overall charge. Familiarity with these ions can significantly speed up the prediction process:
| Polyatomic Ion | Formula | Charge |
|---|---|---|
| Nitrate | NO3- | -1 |
| Sulfate | SO42- | -2 |
| Ammonium | NH4+ | +1 |
| Phosphate | PO43- | -3 |

Recognizing these common ions can help you quickly identify ionic charges in complex compounds.
Use Electron Configuration

The electron configuration of an element tells us how electrons are distributed in its atoms:
- Ionization Energy: Elements with low ionization energy tend to lose electrons, thus forming cations.
- Electron Affinity: Elements with high electron affinity can gain electrons to become anions.
- Stable Electron Configuration: Atoms aim for a stable octet or filled outer shell by losing or gaining electrons.
Charge Balancing in Compounds

In ionic compounds, the total positive charge must equal the total negative charge to achieve charge neutrality:
💡 Note: The sum of the oxidation states of all elements in a neutral compound is zero, while in polyatomic ions, the sum of charges equals the overall ion charge.
- If you’re forming a compound like Na2SO4, Sodium (Na) has a +1 charge, so two Na ions (total charge +2) will balance with one SO4 ion (charge -2).
Practice with Real Examples
Application is key. Here’s how you can practice:
- Exercise 1: Predict the formula of an ionic compound when magnesium (Mg) reacts with chlorine (Cl).
- Exercise 2: Determine the charges of ions in iron(III) chloride (FeCl3).
- Exercise 3: Predict the charges when calcium (Ca) reacts with nitrogen (N) to form a binary compound.
By working through these examples, you’ll develop a deeper understanding of how ions balance out in compounds.
In summary, mastering ionic charges prediction involves a blend of understanding chemical principles, recognizing patterns, and practical application. Familiarity with the periodic table, knowledge of common polyatomic ions, electron configurations, and charge balancing are all critical skills. Through regular practice with real compounds, you'll become adept at predicting ionic charges, enhancing both your problem-solving abilities and your appreciation for the complex beauty of chemistry.
What are the main factors that determine the ionic charge of an element?

+
The main factors influencing the ionic charge of an element include its position in the periodic table, its electron configuration, and its tendency to achieve a stable octet or filled outer shell. Elements with low ionization energy tend to form cations by losing electrons, while those with high electron affinity are more likely to gain electrons and form anions.
Why are some elements capable of forming ions with different charges?

+
Some elements, particularly transition metals, can form ions with different charges due to their variable oxidation states. This variability arises from the complex electron configurations of these elements, allowing for different numbers of valence electrons to be involved in bonding. For example, iron can exist as Fe2+ and Fe3+.
How does knowing the ionic charge help in writing formulas for compounds?

+
Knowing the ionic charges of elements allows you to write balanced chemical formulas. When forming an ionic compound, cations and anions combine in proportions that result in a net charge of zero. This means the positive charges of the cations must equal the negative charges of the anions.
Can the charge of an ion change in different compounds?

+
Yes, the charge of an ion can indeed change depending on the compound it forms. For example, nitrogen can form a -3 ion in ammonium nitrate (NH4NO3) or a -2 ion in nitrogen oxide (NO). The charge is determined by the chemical environment and the balance needed to achieve stability.



