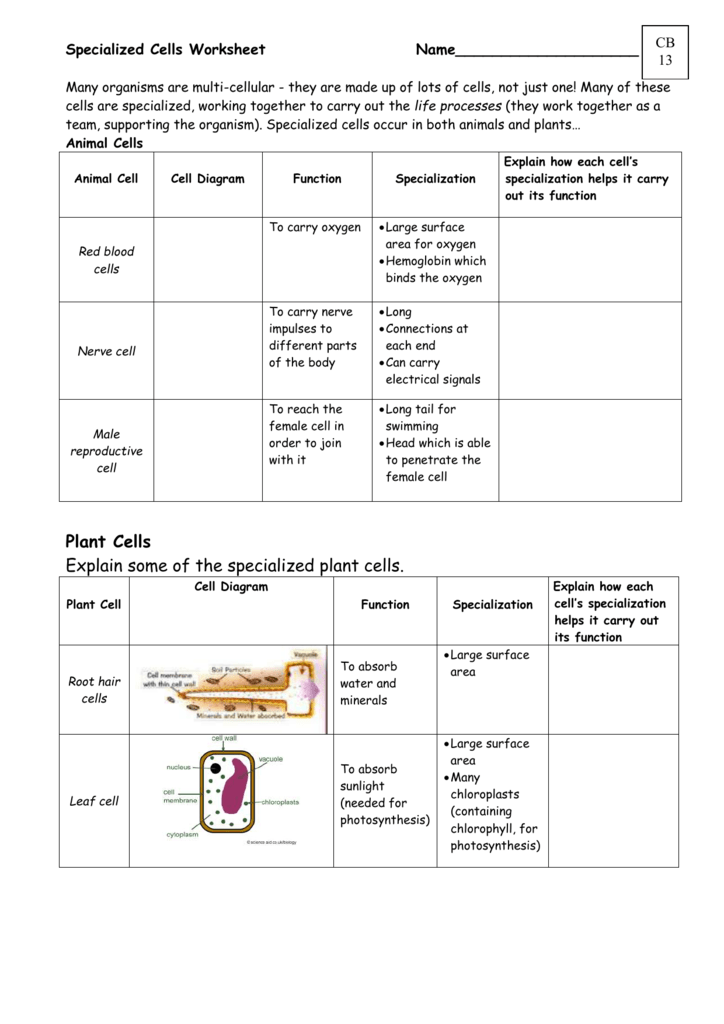
Explore Matter: Physical Properties Worksheet Guide

Matter is the substance that all physical objects are made of, and understanding its physical properties is fundamental in the study of science. Whether you're a student, educator, or an enthusiast of science, diving into the physical properties of matter offers a fascinating journey through the basic principles that govern our universe. In this guide, we'll walk through the key physical properties of matter, explore practical ways to identify these properties, and delve into hands-on activities that can be performed through a worksheet approach to enhance learning and retention.
The Basics of Matter

At its core, matter can exist in three primary states:
- Solid: A state where particles are packed closely together, providing a fixed shape and volume.
- Liquid: Particles have enough energy to move around each other, allowing the substance to take the shape of its container but still maintain a constant volume.
- Gas: Here, particles move freely and rapidly, filling the entire volume of their container without a fixed shape.
The transition between these states, known as changes of state, is a critical part of matter's physical properties.

Physical Properties of Matter

1. Mass and Density

Mass is the amount of matter in an object, while density describes how closely packed that matter is. Density is a fundamental property of matter that can help identify substances. Here’s how you can measure it:
- Find the mass of the object using a balance or scale.
- Measure the volume using graduated cylinders or displacement methods for irregular objects.
- Calculate density using the formula: Density = Mass / Volume.
2. Volume

Volume indicates how much space matter occupies. Liquids can be measured with beakers or graduated cylinders, while for solids, you might use formulas like L x W x H or employ water displacement for irregular shapes.
3. Temperature

Temperature influences the state of matter. You can observe how matter responds to heat, whether it expands, melts, or changes state. Here’s a simple experiment:
- Heat different substances and observe the temperature at which they change state.
4. Melting and Boiling Points

These are distinct properties for each substance:
- Melting Point: The temperature at which a solid turns into a liquid.
- Boiling Point: The temperature at which a liquid turns into a gas.
They can be used to identify substances or explore the purity of materials.
5. Solubility

Solubility is the ability of a substance to dissolve in a solvent, forming a solution. Factors affecting solubility include:
- Temperature
- Nature of the solute and solvent
- Pressure (for gases)
6. Conductivity

This property describes how well a substance can conduct heat or electricity:
- Thermal Conductivity: How well materials conduct heat.
- Electrical Conductivity: How well materials conduct electricity.
7. Hardness and Brittleness

Hardness refers to a material’s resistance to deformation, while brittleness is its tendency to fracture without significant plastic deformation. Here’s how to test them:
- Scratch Test for Hardness: Try scratching one substance with another to compare.
- Breaking Test for Brittleness: Apply force to see if it shatters or deforms.
8. Color and Texture

While these are observable properties, they are less definitive than the others for scientific purposes but can provide clues about the material.
Hands-On Worksheet Activities

To make learning these properties interactive, you can use worksheets:
Activity 1: Density Measurement

Provide students with different materials, such as plastic cubes, metal coins, or ice cubes, along with:
- A balance to measure mass
- Beakers or graduated cylinders for volume measurement
Guide them to calculate density:
- Weigh each object.
- Measure the volume, adjusting for how you measure irregular shapes.
- Calculate and record the density.
📌 Note: Ensure accuracy in measurements, especially for irregular shapes, by using the water displacement method.
Activity 2: Changes in State

Set up an experiment where students can:
- Observe the melting point of different substances (e.g., ice, chocolate, wax).
- Record the temperature changes as substances change from solid to liquid.
📌 Note: Use thermometers to accurately measure the temperature during the state change.
| Substance | State Change | Temperature (°C) |
|---|---|---|
| Ice | Solid to Liquid | 0 |
| Chocolate | Solid to Liquid | 34 - 37 |
| Wax | Solid to Liquid | 40 - 80 |

Activity 3: Solubility Test
Have students experiment with:
- Different solutes like salt, sugar, and sand.
- Varying temperatures to observe how solubility changes.
- Recording results in a table or graph to visualize trends.
Through these hands-on activities, students engage with physical properties in an immersive manner, promoting understanding and retention. These worksheets can be tailored to suit different educational levels, from elementary to high school, adjusting the complexity of the tasks accordingly.
In conclusion, understanding the physical properties of matter through active learning enhances not only the comprehension of these properties but also fosters critical thinking and observational skills. By exploring mass, density, volume, temperature, melting and boiling points, solubility, conductivity, and the texture and color of different substances, students gain a holistic view of the physical world. Such an approach not only prepares them for advanced scientific study but also encourages a lifelong curiosity about how the world around us behaves and interacts at its most fundamental level.
Why is it important to understand physical properties?
+Physical properties allow us to identify, classify, and understand how different materials will behave under various conditions. This knowledge is crucial in fields like materials science, engineering, and chemistry.
How do physical properties differ from chemical properties?
+Physical properties can be observed without changing the chemical composition of a substance, whereas chemical properties describe how substances react with other substances to form new compounds or change their identity.
Can the physical properties of a substance change?
+While the inherent physical properties of a substance are constant, external factors like temperature and pressure can temporarily alter these properties. For example, the volume of a gas changes with temperature, but its density remains a function of its mass and volume.
What are some common errors in measuring physical properties?
+Common errors include inaccurate reading of scales or volume measurements, not considering temperature changes, or failing to properly control experimental conditions like room temperature and pressure.