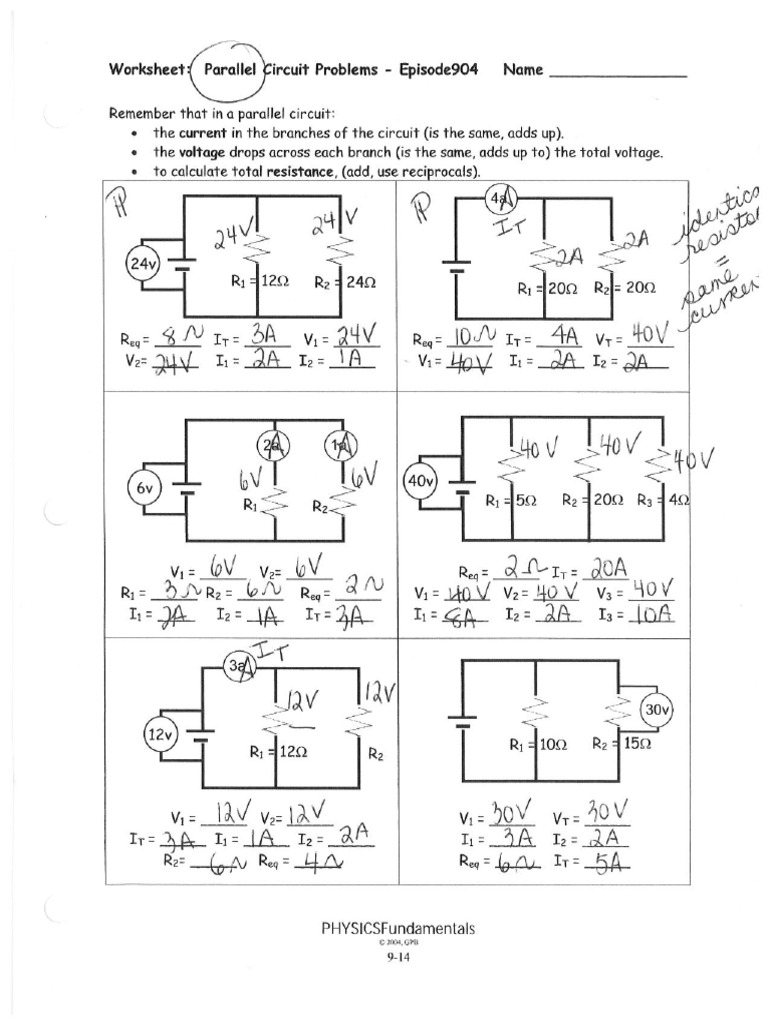
5 Tips for Understanding Heating and Cooling Curves

Heating and cooling curves are fundamental concepts in thermodynamics, chemistry, and engineering, providing insights into how substances change phase under varying temperatures. These curves are not only critical for understanding the behavior of materials but also in practical applications like designing HVAC systems or analyzing food processing techniques. Here are five comprehensive tips to help you better understand and interpret these curves.
1. Identify Key Phases and Transitions

The heating and cooling curve graph essentially depicts temperature changes versus heat added or removed. To effectively read these graphs:
- Horizontal Plateaus: These flat lines indicate phase changes where temperature remains constant despite adding or removing heat. Examples include melting (solid to liquid) or boiling (liquid to gas).
- Sloped Sections: These show temperature changes in a single phase. The steeper the slope, the greater the temperature change for the heat added or removed.
- Interpreting Phases:
- Solid: Below the melting point.
- Liquid: Between melting and boiling points.
- Gas: Above the boiling point.
2. Understand Specific Heat Capacity and Latent Heat
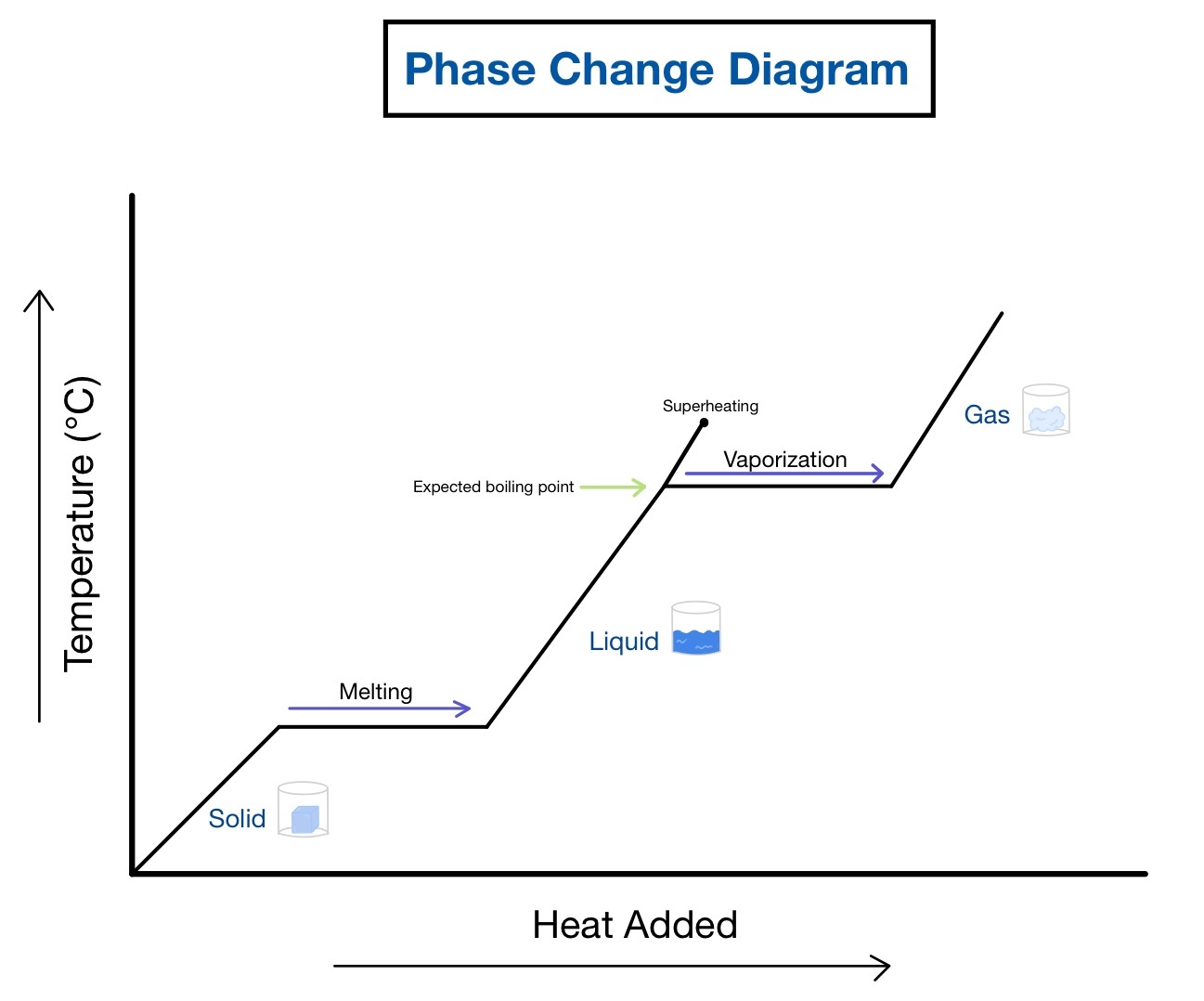
To analyze heating and cooling curves, it's vital to grasp:
- Specific Heat Capacity: This measures the heat required to increase the temperature of one unit mass of a substance by one degree Celsius or Kelvin. It explains the slope of the curve during phase changes where heat goes into changing molecular structure rather than increasing temperature.
- Latent Heat: Energy absorbed or released during phase transitions. Latent heat of fusion (melting) and vaporization (boiling) determine the length of the horizontal plateaus in the curves.
3. Analyze Graphical Data and Applications

Heating and cooling curves can be more than just theoretical constructs; they have practical implications:
- Heat Treating: Engineers use these curves to determine cooling rates for materials like metals to achieve desired properties.
- Chemical Analysis: Chemists can track reaction progress and phase transitions through these graphs.
- Food Industry: Understanding how foods freeze or thaw helps in optimizing preservation techniques.
By examining the slope and plateau in these curves, one can derive valuable data such as specific heats, heat of fusion, or vaporization.
4. Consider Environmental Factors

Environmental conditions can affect heating and cooling curves:
- Ambient Temperature: The surrounding temperature influences the rate at which a substance loses or gains heat.
- Heat Transfer Methods: Conduction, convection, or radiation can change how quickly heat is absorbed or released from a substance.
🗹 Note: The addition of impurities or the presence of external pressure can alter the phase transition temperatures, thus modifying the heating and cooling curve profiles.
5. Calculate and Interpret Data from Curves

Quantitative analysis of heating and cooling curves allows for calculations and predictions:
- Heat Transfer: Using the heat equation Q = mc\Delta T for temperature changes or Q = mL for phase transitions.
- Energy Requirements: For example, determining the energy required to bring water from ice to steam involves summing up the heat absorbed for each segment of the curve.
Here’s a simple table to illustrate this:
| Process | Heat Equation |
|---|---|
| Temperature change (solid/liquid/gas) | Q = mc\Delta T |
| Phase change (Melting/Vaporization) | Q = mL |

By understanding these principles, not only can one interpret these curves but also utilize this knowledge in designing experiments, optimizing industrial processes, or even creating more energy-efficient systems.
Heating and cooling curves offer a window into the nature of substances under thermal stress. By following these five tips, you can master the art of interpreting and using these curves to your advantage. Whether it's for academic purposes or practical applications, understanding these curves provides valuable insights into the behavior of materials as they absorb or release heat.
What are the main applications of heating and cooling curves?

+
Heating and cooling curves are widely used in industries like metallurgy for heat treating materials, in the chemical industry for process analysis, and in food processing to manage preservation techniques.
How do impurities affect heating and cooling curves?

+
Impurities lower the melting point and widen the range over which a substance undergoes phase change, which can be observed as extended plateaus on the curve.
Why is the slope of the heating or cooling curve significant?

+
The slope indicates the rate of temperature change for a given amount of heat added or removed, reflecting the substance’s specific heat capacity.