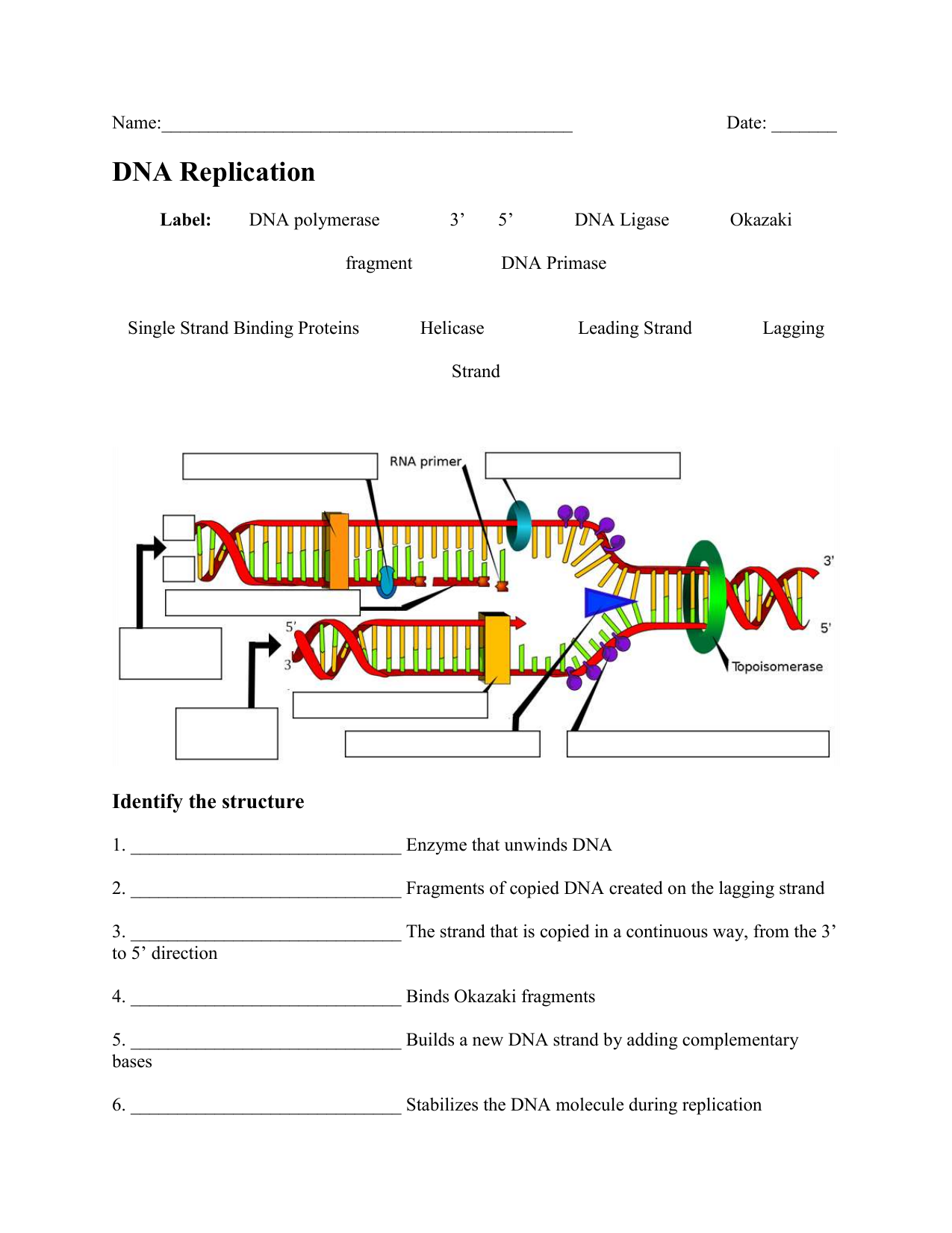
5 Key Photoelectron Spectroscopy Worksheet Answers Revealed

Photoelectron Spectroscopy (PES) is a pivotal technique in the realm of physical chemistry and physics, offering detailed insights into the electronic structure of atoms and molecules. For students and enthusiasts looking to deepen their understanding of PES, mastering the key concepts and worksheet problems is crucial. In this comprehensive blog post, we'll reveal and elucidate 5 key answers from a typical PES worksheet, providing a rich learning experience.
The Basics of Photoelectron Spectroscopy


Before we delve into the worksheet answers, let’s brush up on what PES entails:
- Photoelectron Spectroscopy: It’s an analytical method where photons ionize valence electrons from an atom or molecule.
- Key Components: Photon source, sample, photoelectron analyzer, and a detection system.
- Outcome: Photoelectron energy spectrum, which reflects the binding energies of electrons.
Worksheet Answer #1: Interpreting PES Spectra

In this answer, you’ll learn how to:
- Identify atomic or molecular species based on the electron binding energy peaks.
- Understand the relationship between peak intensity and electron population.
- Interpret the broadening of peaks to infer electron-environment interactions.
🎯 Note: Misinterpretation can occur if one doesn’t account for multiplet splitting or vibrational broadening.
Worksheet Answer #2: Binding Energy Calculations

The calculation of binding energy is essential:
- Use the equation: Binding Energy = Incident Photon Energy - Kinetic Energy of the ejected electron.
- Relate binding energy to ionization energy and understand its significance in electron configurations.
| Electron | Binding Energy (eV) |
|---|---|
| 1s in Neon | 870.2 |
| 2p in Argon | 248.3 |

Worksheet Answer #3: Spectroscopic Selection Rules

Selection rules dictate which transitions are allowed:
- Discuss the significance of angular momentum and spin conservation.
- Understand why certain transitions are forbidden or more probable.
🧭 Note: While PES can detect many transitions, some are not observable due to selection rule restrictions.
Worksheet Answer #4: Chemical Shift in PES

Chemical shift analysis in PES provides information about chemical bonding:
- Identify how electron density affects binding energy.
- Learn to distinguish between different chemical environments of the same element.
Worksheet Answer #5: Experimental Considerations

Understanding experimental challenges and optimization:
- Vacuum conditions to prevent electron scattering.
- Selection of the photon source (X-ray, UV) based on the sample.
- Resolution and sensitivity requirements.
From interpreting spectra to understanding chemical shifts, these five worksheet answers have equipped you with valuable insights into Photoelectron Spectroscopy. This powerful tool not only enhances our understanding of electronic structures but also applies to various fields, from material science to astrochemistry. By mastering PES, you're not just solving problems; you're unlocking the mysteries of matter at an atomic level, which can profoundly impact your journey in science.
What is the main purpose of PES?

+
The main purpose of Photoelectron Spectroscopy is to analyze the electronic structure of matter, particularly to measure the binding energies of electrons in different orbitals, thus revealing information about chemical environments, bonding, and electronic states.
Why do we need high-vacuum conditions in PES?

+
High-vacuum conditions are essential to prevent electron scattering or interactions with atmospheric molecules, which could distort or inhibit the photoelectron spectrum, resulting in loss of valuable data.
How can PES reveal chemical bonding?

+
PES can detect changes in electron binding energies, known as chemical shifts, which occur due to alterations in electron density caused by different chemical bonds or environments. By comparing the binding energies of the same element in different compounds, one can infer bonding characteristics.