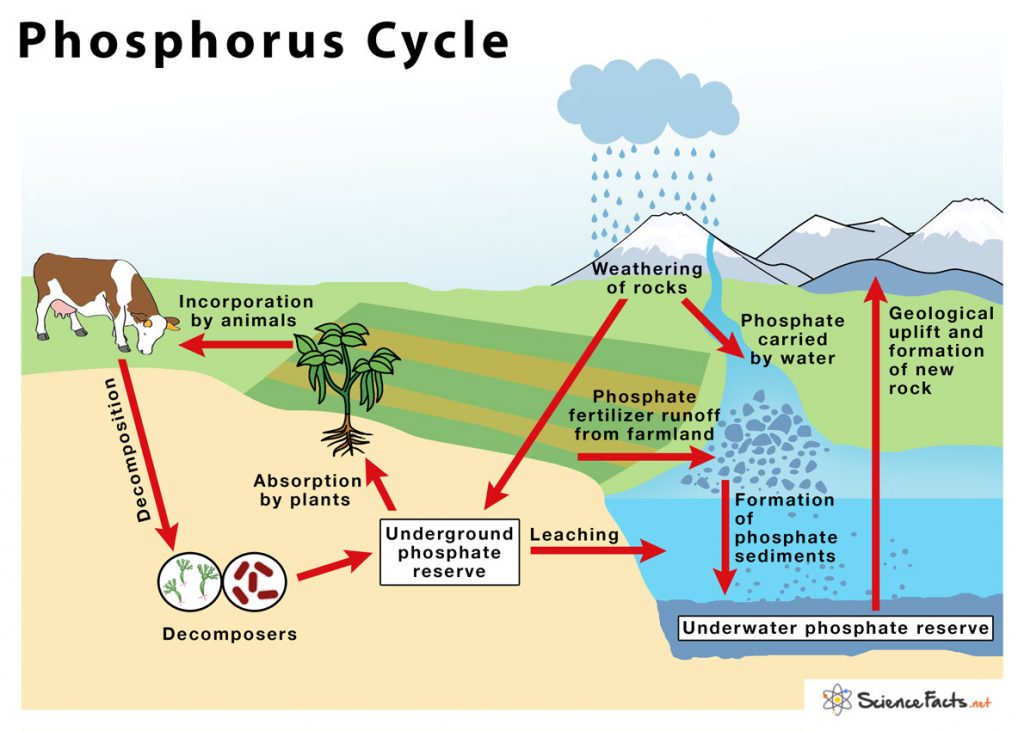
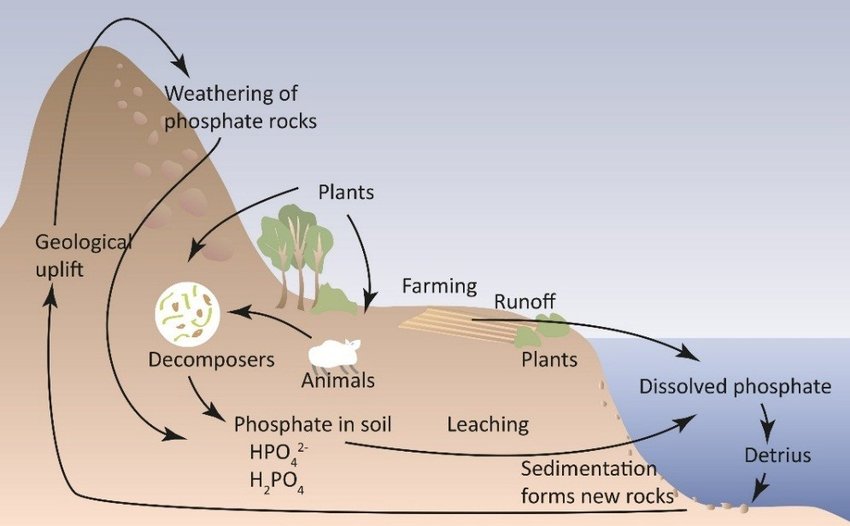
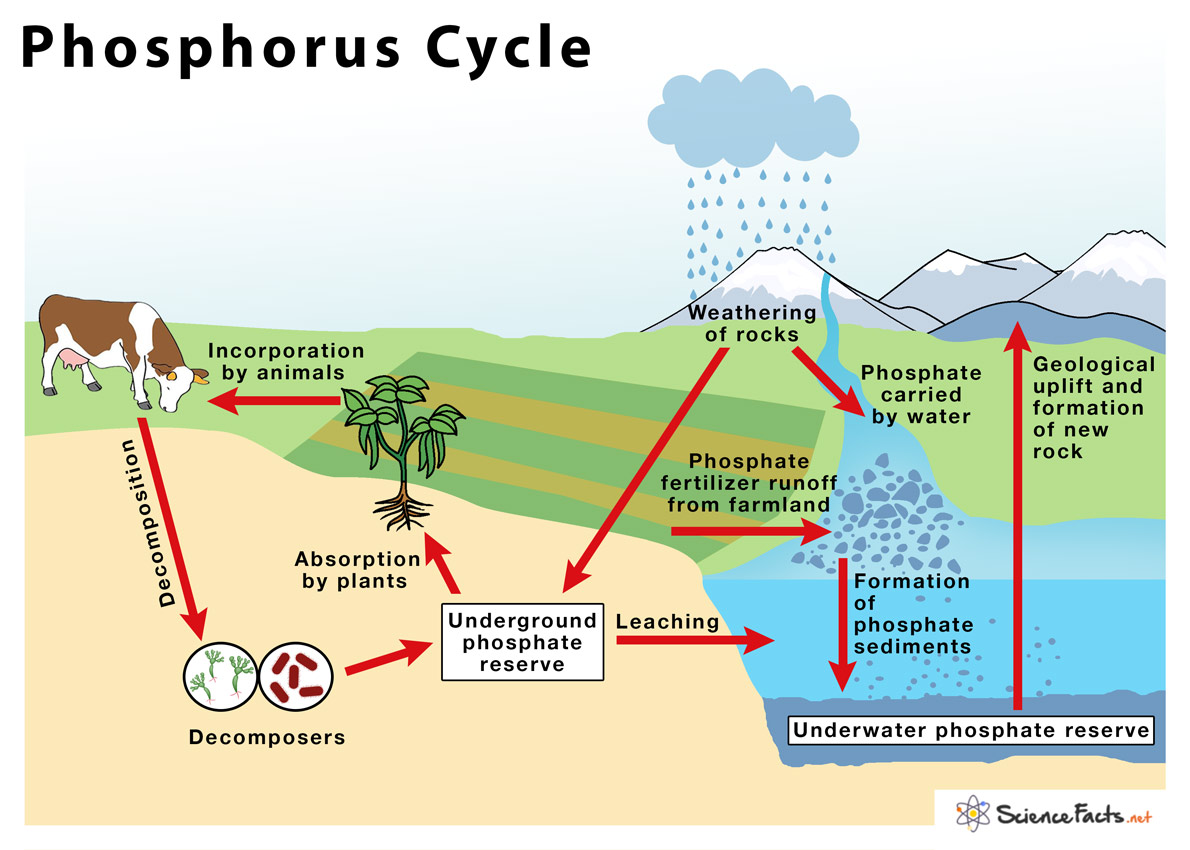
5 Key Steps in the Phosphorus Cycle Explained

The phosphorus cycle plays a vital role in the ecosystem, influencing everything from plant growth to animal health. Unlike other biogeochemical cycles, phosphorus does not have a gaseous component; instead, it cycles primarily through soil, water, and sediment. Here are five key steps in the phosphorus cycle, crucial for understanding how this nutrient sustains life on Earth.
1. Weathering of Rocks


Phosphorus originates from rocks like apatite, where it exists in the form of phosphate minerals. Over time, through the natural process known as weathering, these rocks break down into smaller particles:
- Mechanical weathering: Physical forces like wind, water, and temperature changes break rocks into smaller fragments.
- Chemical weathering: Water and acids from rain or plant roots dissolve the phosphorus-bearing minerals, releasing phosphate ions into the soil.
🌍 Note: Weathering is a slow process, taking millions of years to release phosphorus from rocks into usable forms for plants and animals.
2. Soil Formation and Plant Uptake


Once phosphorus is present in the soil, it’s available for:
- Plant uptake: Plants absorb phosphorus in forms like HPO₄²⁻ or H₂PO₄⁻. This nutrient is essential for energy transfer, nucleic acid, and phospholipid synthesis.
- Soil binding: Phosphorus often binds with iron, aluminum, or calcium ions, forming compounds less available to plants, leading to phosphate fixation.
Plant uptake of phosphorus depends significantly on soil pH:
| Soil pH | Phosphorus Form | Availability to Plants |
|---|---|---|
| Acid | H₂PO₄⁻ | More available |
| Alkaline | HPO₄²⁻ | More available |
| Neutral | Mixed | Moderate |

3. Decomposition and Recycling


When plants and animals die or produce waste:
- Decomposition: Organic matter breaks down, releasing phosphorus back into the soil, where it can again be taken up by plants.
- Nutrient recycling: Bacteria and fungi play crucial roles in this process, converting organic phosphorus into inorganic forms that plants can reabsorb.
♻️ Note: Through decomposition, phosphorus returns to the soil cycle, ensuring nutrient recycling in the ecosystem.
4. Runoff and Water Transport


Excess phosphorus from:
- Fertilizers: Agricultural runoff carries phosphorus from fields into streams, rivers, and eventually oceans.
- Natural sources: Erosion from rocks and soils contributes to the transport of phosphorus to water bodies.
This can lead to:
- Eutrophication, where excess nutrients cause algal blooms, reducing oxygen levels in water and harming aquatic life.
5. Sedimentation and Diagenesis


Phosphorus in water bodies:
- Sedimentation: Phosphorus compounds settle on the bottom of water bodies, forming layers of sediment.
- Diagenesis: Over geological time, this sediment can compact and solidify into rock, trapping phosphorus, or be uplifted to start the cycle again through weathering.
Understanding this cycle is essential for:
- Environmental management to prevent nutrient pollution.
- Sustainable agriculture, where phosphorus availability influences crop production.
This exploration of the phosphorus cycle reveals its importance in maintaining the health of ecosystems. From the weathering of rocks to the complex processes in water bodies, each step is crucial for the circulation of this essential nutrient. By understanding these steps, we can appreciate the delicate balance that sustains life and the importance of managing phosphorus efficiently to prevent ecological disruption.
Why is the phosphorus cycle important?

+
The phosphorus cycle is crucial because phosphorus is an essential element for life, needed for DNA, RNA, ATP, and phospholipids in cell membranes. Its availability affects plant growth, which in turn supports the entire food chain, influencing ecosystem health and productivity.
What are the human impacts on the phosphorus cycle?
+
Humans impact the phosphorus cycle primarily through agriculture (use of fertilizers), mining of phosphorus-rich rock, and waste disposal. These activities can lead to an overload of phosphorus in water systems, causing eutrophication, oxygen depletion, and harm to aquatic life.
Can phosphorus be recycled?
+
Yes, phosphorus can be recycled through natural decomposition processes where it returns to the soil, and through human efforts like composting and waste treatment technologies that recover phosphorus from sewage sludge.
How does soil pH affect phosphorus availability?
+
Soil pH greatly influences phosphorus availability. In acidic soils, phosphorus tends to bind with aluminum and iron, while in alkaline soils, it combines with calcium, both leading to less available forms of phosphorus for plants. The optimum pH range for phosphorus availability is usually between 6.0 and 7.0.