Phase Diagram Worksheet: 5 Key Answers Revealed

In the realm of thermodynamics, understanding how materials behave under different conditions is crucial for fields ranging from material science to environmental engineering. One powerful tool scientists and engineers use to predict and analyze the behavior of a substance under varying conditions of temperature and pressure is known as a phase diagram. Here are five key insights that can unlock the mysteries of phase diagrams, providing a deeper understanding for students, researchers, and enthusiasts.
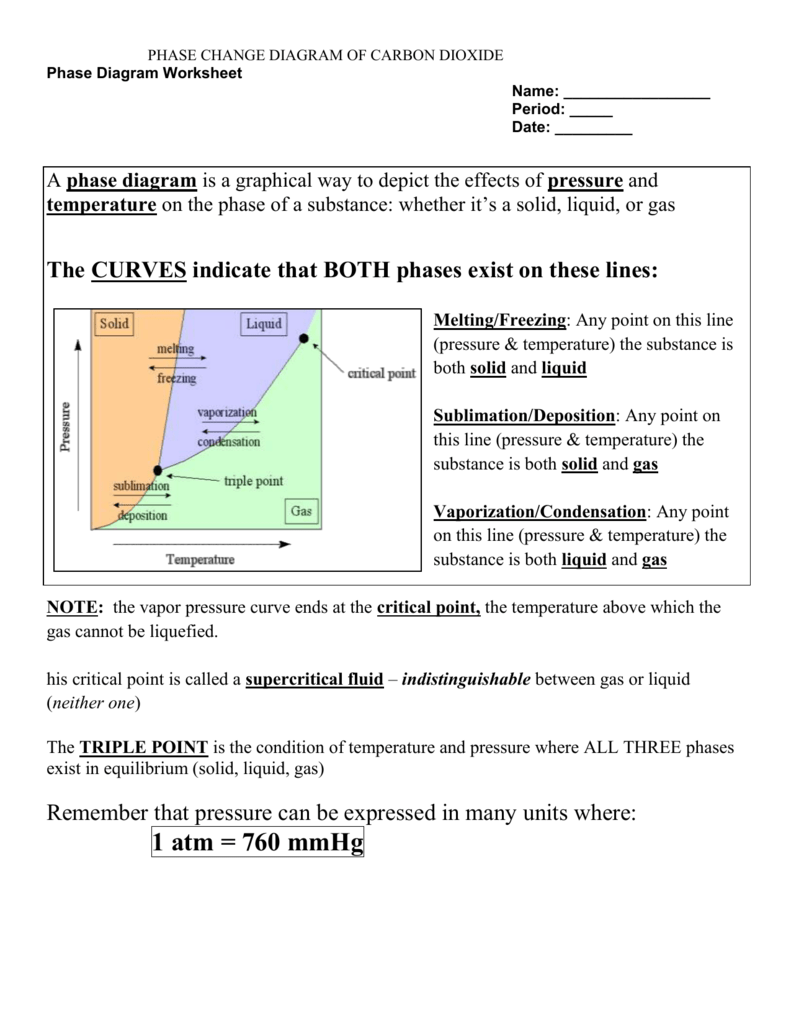
Understanding Phase Diagrams

A phase diagram is a graphical representation that shows the stability of different phases of a material at different temperatures and pressures. Here's what you need to know:
- Phases: Solid, liquid, gas, and sometimes plasma, supercritical fluid, or other exotic states.
- Lines or Boundaries: These separate different phases; crossing one means a phase transition occurs.
- Critical Point: The end of the liquid-gas line where distinctions between these phases disappear.
- Triple Point: The unique condition where all three common phases coexist in equilibrium.
1. Reading the Phase Diagram

Interpreting a phase diagram involves understanding:
- Horizontal and Vertical Axes: Temperature and pressure, respectively, are the axes on which the graph is plotted.
- Phase Regions: Areas within the diagram where a substance exists in a specific phase.
- Equilibrium Lines: These show where two phases can exist in equilibrium.
📚 Note: Always start with understanding what the axes represent to correctly interpret the phase diagram.
2. Phases and Phase Transitions

Each phase in a phase diagram represents:
- Solid Phase: The substance is at a low temperature or high pressure.
- Liquid Phase: Intermediate conditions where the substance is in a fluid state.
- Gaseous Phase: High temperatures or low pressures where molecules move freely.
To transition between phases:
- From solid to liquid, the process is called melting or fusion.
- Liquid to gas transition is vaporization or evaporation.
- The reverse is condensation and solidification or freezing, respectively.
3. Critical Points and Beyond

The critical point on a phase diagram holds special significance:
- It marks the condition where the liquid and gas phases are indistinguishable.
- Beyond this point, the substance enters a supercritical state.
At the critical point, the properties like density, viscosity, and specific heat capacity become the same for both liquid and gas:
| Property | Before Critical Point | At Critical Point |
|---|---|---|
| Density | Different (liquid > gas) | Same |
| Viscosity | Liquid > Gas | Intermediate |
| Specific Heat Capacity | Liquid > Gas | Same |

⚗️ Note: Supercritical fluids have unique properties that make them excellent solvents for industrial processes.
4. Triple Point and Eutectic Systems

Another fascinating feature of phase diagrams is the triple point:
- The substance can exist in all three common phases simultaneously.
- It's a fixed point, unique for each substance.
Eutectic systems involve:
- The formation of two solid phases directly from a liquid phase.
- The lowest melting temperature of any mixture of the two components.
🔬 Note: Understanding the triple point helps in calibrating thermometers, where substances like water are used for precise temperature measurements.
5. Applications of Phase Diagrams

The practical applications of phase diagrams include:
- Material Selection: Choosing materials based on their phase behavior.
- Phase Engineering: Manipulating conditions to achieve desired phase properties.
- Industrial Processes: Crucial for distillation, extraction, and material processing.
They play a significant role in various industries, from metallurgy to pharmaceuticals, where knowing the phase transitions of substances can:
- Optimize synthesis conditions.
- Predict material performance under different operating conditions.
- Guide the formulation of mixtures.
Ultimately, the study of phase diagrams not only reveals the fundamental behavior of substances but also empowers engineers and scientists to harness these behaviors for practical applications. Understanding these key elements allows us to predict how materials will react under different pressures and temperatures, facilitating innovations in material design and process engineering.
What are the main phases represented in a phase diagram?

+
The main phases are solid, liquid, and gas. Other phases like plasma or supercritical fluid might also be present depending on the substance.
Why is the critical point important in a phase diagram?

+
The critical point marks the conditions at which the liquid and gas phases become indistinguishable, leading to a supercritical fluid state with unique properties that have industrial applications.
How do phase diagrams help in industrial applications?

+
Phase diagrams help in selecting materials, engineering desired properties, optimizing synthesis conditions, and guiding the formulation of mixtures for various industrial processes.
Can you explain the concept of a triple point?
+
The triple point is where a substance can exist in solid, liquid, and gas phases simultaneously at a specific temperature and pressure. It’s used for precise calibration of thermometers.
What are the benefits of understanding phase transitions for material design?

+
Knowing how materials transition from one phase to another allows for better material selection, optimization of manufacturing processes, and development of new materials with desired properties.



